Prototyping Crystals (Grown)
So I collected most of my recipes below in a catalogue. Were all created at the same time to help me measure growth rate!

Crystals Catalogue by BOR by Batool Alrushdan
Grown
during this research I had developed "crystals catalogue" for deep analysis and studies with varying factors / recipes. However I maintained "Controlled environment" and had constant amount of water as solvent (to be capable of truly compare and measure the crystal growth rate)
Solvent: 10 mm of Water
The solute:
- Alum - Local supplier
- Alum - International supplier
- Borax - Local supplier
- Salt - table salt
Modifiers: - M. - pigments - Thermochromic Pigments
Some Terms were worth understanding for the processes
- Measurement of growth rate Methods used for the measurement of crystal growth rates are either a) direct measurement of the linear growth rate of a chosen crystal face or b) indirect estimation of an overall linear growth rate from mass deposition rates measured on individual crystals or on groups of freely suspended crystals.
Not to mention Visual documentation of the crystals formation.
Face growth rates. Different crystal faces grow at different rates and faces with a high value of s grow faster than faces with low values. Changes in growth environment such as temperature, supersaturation pH, and impurities can have a profound effect on growth, and differences in individual face growth rates give rise to habit changes in crystals. For the measurement of individual crystal-face growth-rates, a fixed crystal in a glass cell is observed with a travelling microscope under precisely controlled conditions of solution temperature, supersaturation and liquid velocity. The solution velocity past the fixed crystal is often an important growth-determining parameter, sometimes responsible for the so-called size-dependent growth effect often observed in agitated and fluidised-bed crystallisers. Large crystals have higher settling velocities than small crystals and, if their growth is diffusion-controlled, they tend to grow faster. Salts that exhibit solution velocity dependent growth rates include the alums, nickel ammonium sulphate, and potassium sulphate, although salts such as ammonium sulphate and ammonium or potassium dihydrogen phosphate are not affected by solution velocity.
Overall growth rates. In the laboratory, growth rate data for crystalliser design can be measured in fluidised beds or in agitated vessels, and crystal growth rates measured by growing large numbers of carefully sized seeds in fluidised suspension under strictly controlled conditions. A warm undersaturated solution of known concentration is circulated in the crystalliser and then supersaturated by cooling to the working temperature. About 5 g of closely sized seed crystals with a narrow size distribution and a mean size of around 500 μm is introduced into the crystalliser, and the upward solution velocity is adjusted so that the crystals are maintained in a reasonably uniform fluidised state in the growth zone. The crystals are allowed to grow at a constant temperature until their total mass is some 10 g, when they are removed, washed, dried, and weighed. The final solution concentration is measured, and the mean of the initial and final supersaturations is taken as the average for the run, an assumption which does not involve any significant error because the solution concentration is usually not allowed to change by more than about 1 per cent during a run. The overall crystal growth rate is then calculated in terms of mass deposited per unit area per unit time at a specified supersaturation.
- The nucleation rate: which is a convenient synthesis of terms that describes how many nuclei of critical size form on a substrate per unit area, per unit time. Nuclei can grow through direct impingement of gas-phase atoms, but this is unlikely in the earliest stages of film formation when nuclei are spaced far apart.
Copper
Optic Fiber


- I was talking a picture every time interval to check the crystal growth rate
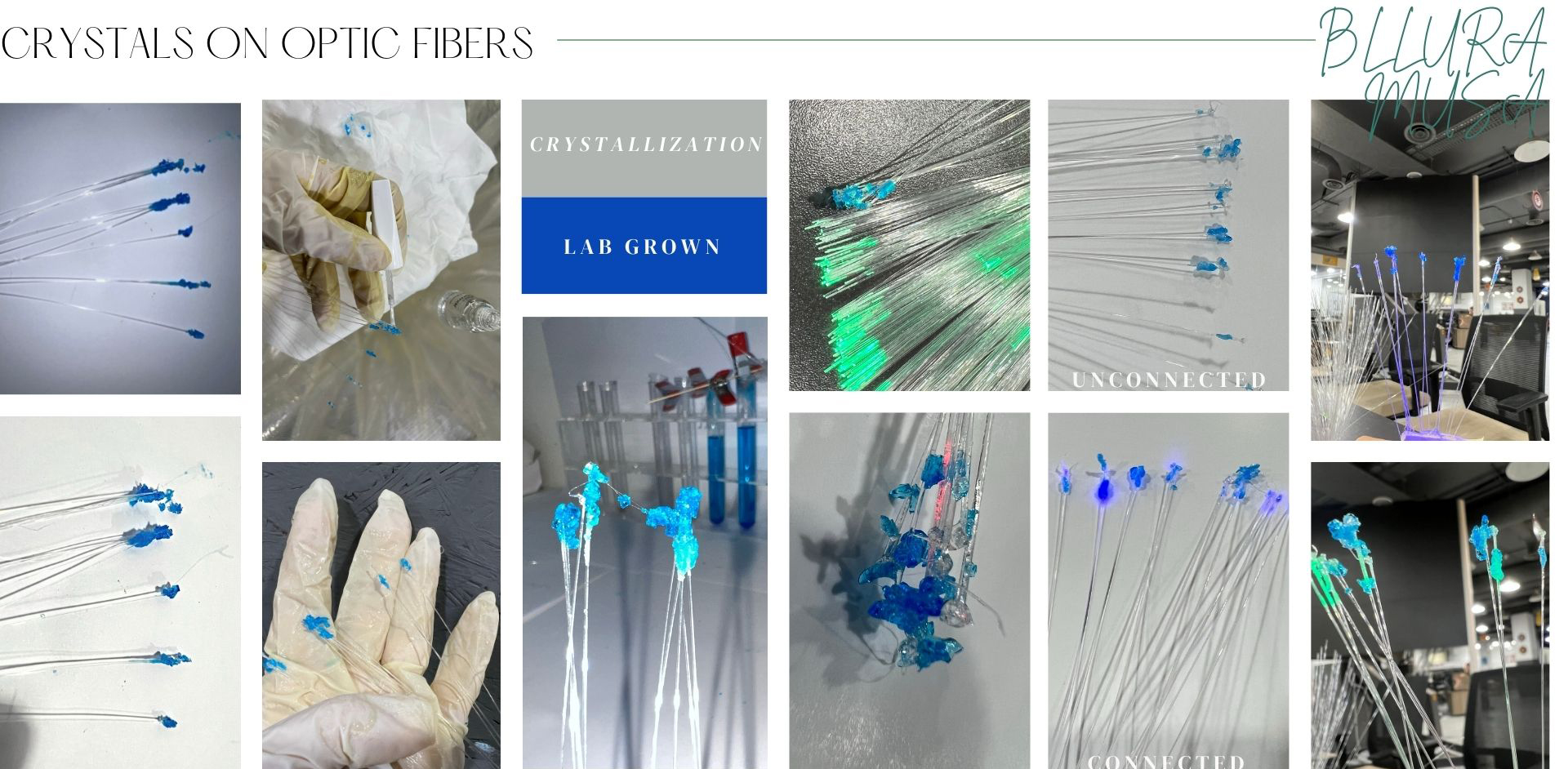
-
These were the results of the grown copper crystals on the optic fiber in test tubes (after isolating the Optic fibers)
-
connected to source of lighting to test them
- RGB was coded to change color so I was documenting several colors of the light under the crystals (Turquoise , Red, Dark blue )

Borax
Optic Fiber


Alum
Methodology
Growing Single crystal
(Research according to The University of Otago which is ranked in the top 1% of universities in the world) - Chemistry
Alum, or potassium aluminium sulfate, is a chemical compound with the formula KAl(SO4).12H2O. It has been known for thousands of years and is used in various ways - in taxidermy, as a deodorant, in pickling food, in fire extinguishers. Probably its most important use is in the purification of drinking water.
It forms beautiful, regular octahedral crystals such as the one shown here. Normally alum crystals are colourless, but if the ‘normal’ alum (KAl(SO4).12H2O) is mixed with ‘chrome alum’ (KCr(SO4).12H2O) then the crystals will have a purple colour.
To grow large single crystals of alum, you first need to grow what is called a seed crystal. This should be a single crystal, but just a small one. (It is normally better for the Alum to crystallise as lots of small crystals rather than one big one). A suitable seed crystal is then suspended in a supersaturated solution of alum. A supersaturated solution is one which has more alum dissolved that is normally possible. Such a solution wants to get rid of the extra dissolved solid– if you are careful then it will do this by adding solid alum to your seed crystal, making it grow bigger.
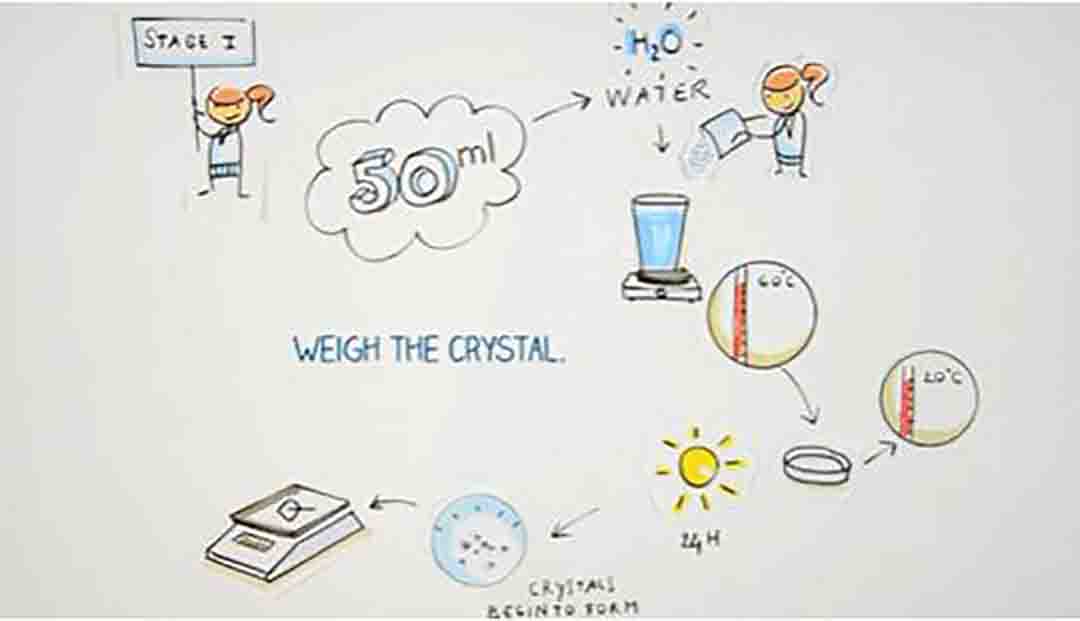
Step 1:
Make a supersaturated solution of alum. Do this by adding alum to hot water until no more will dissolve.
The exact amount of alum will vary depending on the temperature of the water but it will be about 15 g/25 mL.
Step 2:
crystal-watchglass Pour some of the solution into a shallow evaporating dish and leave in a cool, dark, undisturbed cupboard overnight. As the solution cools and begins to evaporate, small seed crystals will form in the evaporating dish.

Step 3:
seed-crystal Select your seed crystal. Find the nicest one by looking at them with a magnifying glass – it should be a single crystal (not two or more stuck together) and transparent, with sharp edges and corners. Carefully remove your seed crystal using tweezers (try not to touch the crystal with your fingers) and the tie it to a length of fine nylon fishing line, tying a knot around the seed crystal. Then tie the other end of the fishing line around a pencil or similar.
Step 4:
Make another supersaturated solution of alum in a clean beaker or jar. Suspend the seed crystal in the saturated solution Make sure it is not touching the sides or the bottom of the container. Cover with aluminium foil or filter paper and leave in an undisturbed place. It is vital that you minimise how much you move the crystal. It is also important that the temperature remains as constant as possible – you could place the beaker in a chilly bin or similar to help with this. If the solution heats up (because the heaters in your classroom turn on unexpectedly) then your crystal may redissolve!
Step 5:
suspended-crystal Check on the crystal each day. There should be visible growth of the crystal. If the crystal stops growing or decreases in size, the solution is no longer saturated so a new one will need to be made. To do this first carefully remove your crystal, then warm the solution and dissolve some more alum in it. Then, when it has cooled back to room temperature, return the crystal to the solution. You may have to do this more than once, until you run out of Alum
Step 6:
When the crystal has grown as large as it can, then carefully remove it, let it dry on some paper towel.
My own Conclusion: - add to the mixture until no more dissolves so proportions depends on you! - stir the mixture on heat while doing That - remove lets it cool until 20 degrees - now suspend the seed crystal - cover to prevent environmental changes (temperature) temperature must be controlled as much as we can and dust ... so best advice to store growing crystal(the suspended with the jar covered) inside picnic cooler or foam box
you can always remove repeat the steps above by removing it and dry it with paper towel and place it in another more saturated solution for more growth and measure its weight all alone
another growth of single crystal by ThoughtCo:
Grow Alum Crystals That Resemble Simulated Diamonds

Alum is found in the spices section of the grocery store. That little jar contains small white crystals that, with a bit of time and effort, grow a big alum crystal that looks a bit like a diamond. It only takes about an hour to grow small alum crystals, but getting a big crystals takes days to weeks.
Grow a Big Alum Crystal
Alum crystals are colorless, non-toxic crystals that are easy to grow.
Large crystals somewhat resemble diamonds, although they are much softer than the gemstones.
Expect growing a large crystal to take days or a couple of weeks.
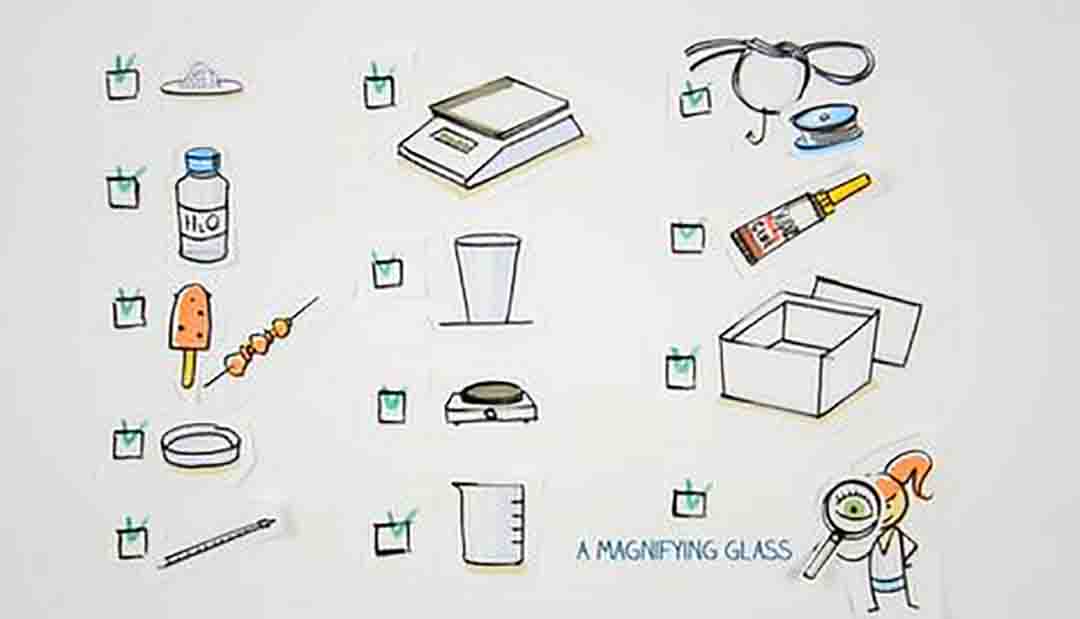
What You Need for Alum Crystals All you need to grow alum crystals are alum, hot water, and a container. Choose a clear container so you can watch the crystals grow. While not strictly necessary, it helps having a way to tie and suspend a crystal in the liquid. This helps it keep an ideal shape. A coffee filter or paper towel keeps dust out of your project, while still allowing good air circulation.
FEATURED VIDEO Grow Alum Crystals 1/2 cups hot tap water 2-1/2 tablespoons alum nylon fishing line pencil, ruler, or knife 2 clean jars spoon coffee filter/paper towel There are actually a few different kinds of alum. The edible one in the grocery store is potassium alum. It grows clear crystals. Other types of alum include sodium, ammonium, selenium, and chrome alum. Chrome alum grows deep purple crystals. If you have access to the other chemicals, feel free to combine them to see what colors you get. But, check the labels for safety information. Some types of alum are non-toxic, but others are irritants and not edible.
Grow the Crystals Pour 1/2 cup of hot tap water into a clean jar. Slowly stir in alum, a little at a time, until it stops dissolving. Don't add the whole amount; just enough to saturate the water. Loosely cover the jar with a coffee filter or paper towel (to keep dust out) and allow the jar to sit undisturbed overnight. The next day, pour the alum solution from the first jar into the clean jar. You will see small alum crystals at the bottom of the jar. These are 'seed' crystals that you will use to grow a big crystal. Tie nylon fishing line around the largest, best-shaped crystal. Tie the other end to a flat object (e.g., popsicle stick, ruler, pencil, butter knife). You will hang the seed crystal by this flat object into the jar far enough so that it will be covered in liquid, but won't touch the bottom or sides of the jar. It may take a few tries to get the length just right. When you have the right string length, hang the seed crystal in the jar with the alum solution. Cover it with the coffee filter and grow a crystal! Grow your crystal until you are satisfied with its size. If you see crystals starting to grow on the sides or bottom of your jar, carefully remove your crystal, pour the liquid into the clean jar, and put the crystal in the new jar. Other crystals in the jar will compete with your crystal for alum, so it won't be able to get as big if you let these crystals grow. Solving Common Problems The most common problem people experience growing alum crystals is that crystals not growing. If you don't see crystal growth within a day or two, there isn't enough alum in the liquid. Gently heat the liquid over a stove or in the microwave and try adding more alum powder. Crystals only grow if the solution is saturated. This is the point where no more solid dissolves. What You Need for Alum Crystals All you need to grow alum crystals are alum, hot water, and a container. Choose a clear container so you can watch the crystals grow. While not strictly necessary, it helps having a way to tie and suspend a crystal in the liquid. This helps it keep an ideal shape. A coffee filter or paper towel keeps dust out of your project, while still allowing good air circulation. FEATURED VIDEO Grow Alum Crystals 1/2 cups hot tap water 2-1/2 tablespoons alum nylon fishing line pencil, ruler, or knife 2 clean jars spoon coffee filter/paper towel
There are actually a few different kinds of alum. The edible one in the grocery store is potassium alum. It grows clear crystals. Other types of alum include sodium, ammonium, selenium, and chrome alum. Chrome alum grows deep purple crystals. If you have access to the other chemicals, feel free to combine them to see what colors you get. But, check the labels for safety information. Some types of alum are non-toxic, but others are irritants and not edible.
Grow the Crystals Pour 1/2 cup of hot tap water into a clean jar. Slowly stir in alum, a little at a time, until it stops dissolving. Don't add the whole amount; just enough to saturate the water. Loosely cover the jar with a coffee filter or paper towel (to keep dust out) and allow the jar to sit undisturbed overnight. The next day, pour the alum solution from the first jar into the clean jar. You will see small alum crystals at the bottom of the jar. These are 'seed' crystals that you will use to grow a big crystal. Tie nylon fishing line around the largest, best-shaped crystal. Tie the other end to a flat object (e.g., popsicle stick, ruler, pencil, butter knife). You will hang the seed crystal by this flat object into the jar far enough so that it will be covered in liquid, but won't touch the bottom or sides of the jar. It may take a few tries to get the length just right. When you have the right string length, hang the seed crystal in the jar with the alum solution. Cover it with the coffee filter and grow a crystal! Grow your crystal until you are satisfied with its size. If you see crystals starting to grow on the sides or bottom of your jar, carefully remove your crystal, pour the liquid into the clean jar, and put the crystal in the new jar. Other crystals in the jar will compete with your crystal for alum, so it won't be able to get as big if you let these crystals grow. Solving Common Problems The most common problem people experience growing alum crystals is that crystals not growing. If you don't see crystal growth within a day or two, there isn't enough alum in the liquid. Gently heat the liquid over a stove or in the microwave and try adding more alum powder. Crystals only grow if the solution is saturated. This is the point where no more solid dissolves.
Crystal Growing Tips - You can use sewing thread or other string instead of nylon fishing line, but crystals will grow on the entire length of the submerged string. Crystals don't adhere to nylon, so if you use it, you can get bigger and better crystals. - Alum is an ingredient used to make pickles. It makes them crispy. - Don't worry if you don't want to bother with the string! Crystals grow just fine on the bottom of the container. Use a spoon to scrape crystals away from each other so they won't grow together. The shape of crystals growing on a flat surface differs from the shapes that form when crystals are suspended.
all the steps above to get one crystal extra dramatic lots of effort for just one crystal taking several days so lets jump to the overnight options:
Salt crystals
Table salt, also known as sodium chloride, is a crystal (a symmetrical solid substance made entirely of the same material). You can see the shape of a salt crystal under a microscope. Growing salt crystals is fun and easy; the ingredients are right in your kitchen, the crystals are non-toxic, and no special equipment is required.
How to Grow Salt Crystals It takes very little work to start the process of growing salt crystals, though you will need to wait a few hours or days to see the results, depending on the method you use.
Salt Crystal Materials
- table salt (sodium chloride)
- water
- clean clear container
- a piece of cardboard (optional)
- string and pencil or butter knife (optional)
Procedures
Stir salt into boiling hot water until no more salt will dissolve (crystals start to appear at the bottom of the container). Be sure the water is as close to boiling as possible. Hot tap water is not sufficient for making the solution.
Quick Crystals: If you want crystals quickly, you can soak a piece of cardboard in this supersaturated salt solution. Once it is soggy, place it on a plate or pan and set it in a warm and sunny location to dry out. Numerous small salt crystals will form.
Perfect Crystals: If you are trying to form a larger, perfect cubic crystal, you will want to make a seed crystal. To grow a big crystal from a seed crystal, carefully pour the supersaturated salt solution into a clean container (so no undissolved salt gets in), allow the solution to cool, then hang the seed crystal in the solution from a pencil or knife placed across the top of the container. You could cover the container with a coffee filter if you like.
Set the container in a location where it can remain undisturbed. You are more likely to get a perfect crystal instead of a mass of crystals if you allow the crystal to grow slowly (cooler temperature, shaded location) in a place free of vibrations.
Tips for Success Experiment with different types of table salt. Try iodized salt, un-iodized salt, sea salt, or even salt substitutes. Try using different types of water, such as tap water compared with distilled water. See if there is any difference in the appearance of the crystals. If you are trying for the 'perfect crystal' use un-iodized salt and distilled water. Impurities in either the salt or water can aid dislocation, where new crystals don't stack perfectly on top of previous crystals. The solubility of table salt (or any kind of salt) increases greatly with temperature. You'll get the quickest results if you start with a saturated saline solution, which means you want to dissolve salt in the hottest water available. One trick to increase the amount of salt you can dissolve is to microwave the salt solution. Stir in more salt until it stops dissolving and starts to accumulate at the bottom of the container. Use the clear liquid to grow your crystals. You can filter out the solids using a coffee filter or paper towel.
Summary of growing crystals
this is personal simple summary/brief of tip/notes and important observations of growing crystals locally wherever you are
-
Crystals grew the best (size wise, geometry wise and how fast) when i grew them in a glass jar ( that glass container was filled with distilled water put directly on the oven and let it boil and then borax was added with correct calculations so was super saturated then the optic fibers were dipped in it (before dipping them they were prepared by insulating their tips with super glue and some while insulating was gluing some crystals leftover from deadsea to their tips so i guess these acted as my seed crystals ))
-
when I was growing the headpiece however the solution wasn't super saturated … I made it in purpose like that as I wanted to slow down the growth rate of the crystals in order to be able to take the glass tank to the deadsea to shoot it otherwise crystals would have formed before even reaching there however there was Growth which is fair enough
-
once i tried growing them directly from the stainless steel pot and it was somehow a failure (crystals didnt seem to like the stainless steel pot although was sterilized like i put the distilled water put directly on the oven and let it boil and then borax was added with correct calculations so was super saturated so repeated all the above BUT in stainless steel pot not in a glass... another factor i actually didn't dip any object for the crystals t grow unlike the previous experiments and was thinking ill optain few crystals at the bottom that i can collect )
Sometimes I used the onions dye bath to grow crystals instead of clear water to gain this off-white especially the one on the Main Front piece/ strophium.(but everything in the recipe is the same)
Post process
Polish!
Final Dress
 Firstly, I started with choosing the shades of Tulle the works the best with my color palette so I took the pictures of the deadsea you see here with me to the shop and I started putting different shades on top of each other as I was convinced to truly match and create the perfect Camouflage of the dead sea ( as you can see the near salts have the white/off-white color tone while the new warmer water has more of a Turquoise color and the far/colder water has the navy darker blue color)
Firstly, I started with choosing the shades of Tulle the works the best with my color palette so I took the pictures of the deadsea you see here with me to the shop and I started putting different shades on top of each other as I was convinced to truly match and create the perfect Camouflage of the dead sea ( as you can see the near salts have the white/off-white color tone while the new warmer water has more of a Turquoise color and the far/colder water has the navy darker blue color)
-
At the end I picked the perfect match of Top/ Upper Tulle Turquoise - matching color nearer water color of the dead sea
-
The underneath Tulle Navy blue (way darker) - matching the far end of the sea
as I thought I would leave the Navy Blue shade of tulle untouched, while ** grow attach 3D printed Crystals on the Turquoise Tulle plus grow the crystals on!

- This Alum recipe - I started firstly by growing seed crystals as you see in the first 2 pictures then I harvested/filtered them and filtered the solution.
- I cleaned the fish Tank then use water bath for further clean it
- I kept it in the water bath after fully dried
- Heated the previous solution but added more Alum to it making it more condensed/saturated
- Filtered the solution into the Tank Again
- left it to boiling
- Added the Turquoise Tulle after attaching the 3D printed crystals as well as the real seed crystals
- Covered it (to prevent insects/dust from mixing)
- Left it for few days!

- Here I took the dress from the fish tank as you can see with lovely crystals grown on that Turquoise Tulle
- I put it to dry on the sun
simple references
Resources
Alum crystals according to The University of Otago
Alum crystals according to ThoughtCo