9. Textile as scaffold¶
November 17th, 2020 by Anastasia Pistofidou
Technical textiles have various applications, among which agrotech, building, clothes, geotech, sports. In this class we studied how to use textiles for composites, polymerisation, solidification, fabric formwork, crystallization, composites and biocomposites, agglomerates.
This class focused on exploring techniques and applications of technical textiles in the industry. It introduced the concept of designing custom processes that require the design of a set of tools, processes and workflow.
Research¶
- Entry Paradise Pavilion
- Digital Fabrications. Architectural and Material Techniques. Lisa Iwamoto
- Between the Sheets
- Elisa Strozyk
Assignment¶
Leather molding - Leather Sample 1¶
Step 1: Designing the mould¶
Rhino+Grasshopper
To start this week's assignment I designed and 3d modelled a volume to use as a mould for the leather sample. I decided to design it using Grasshopper, following this Metaball Tutorial.
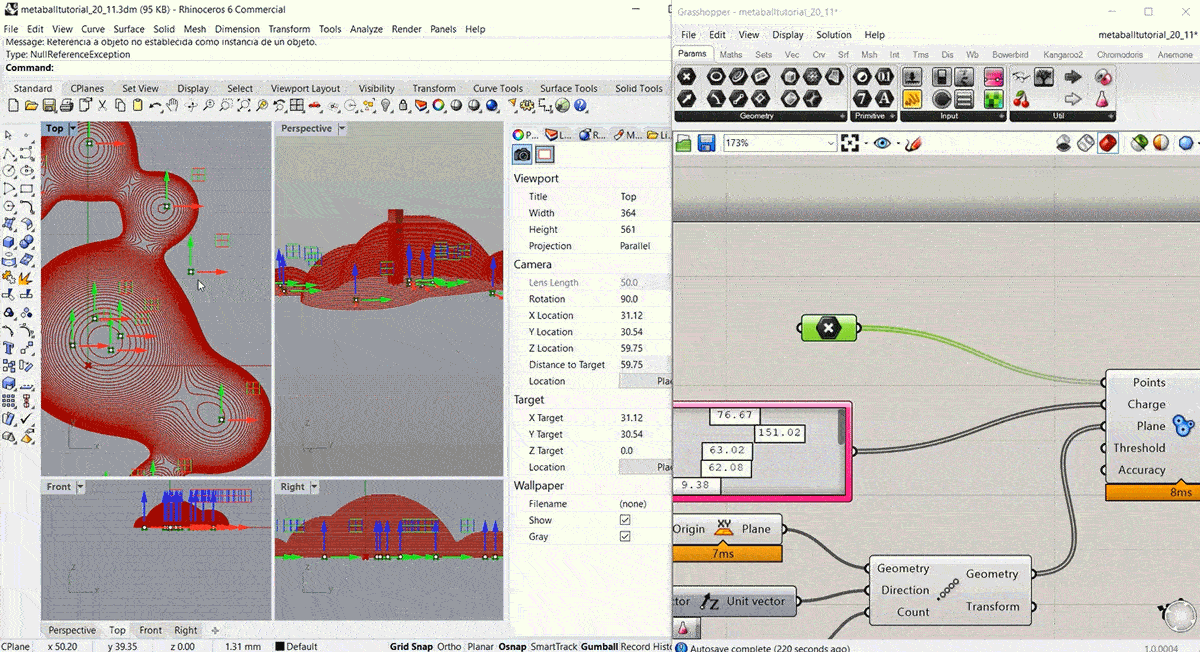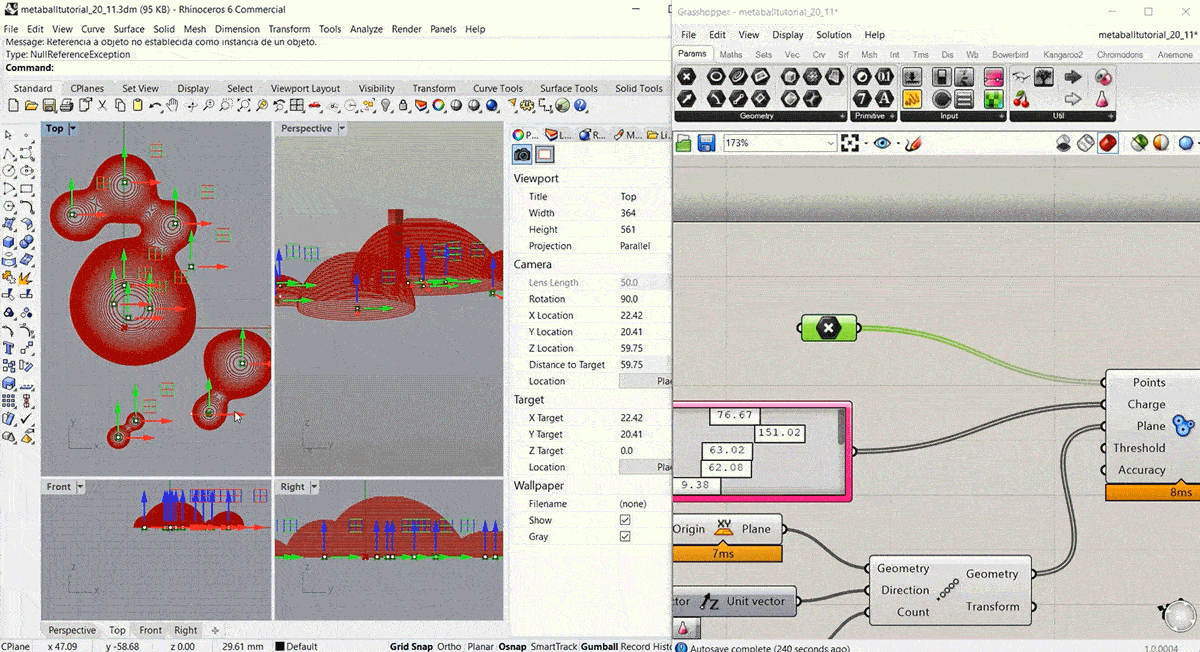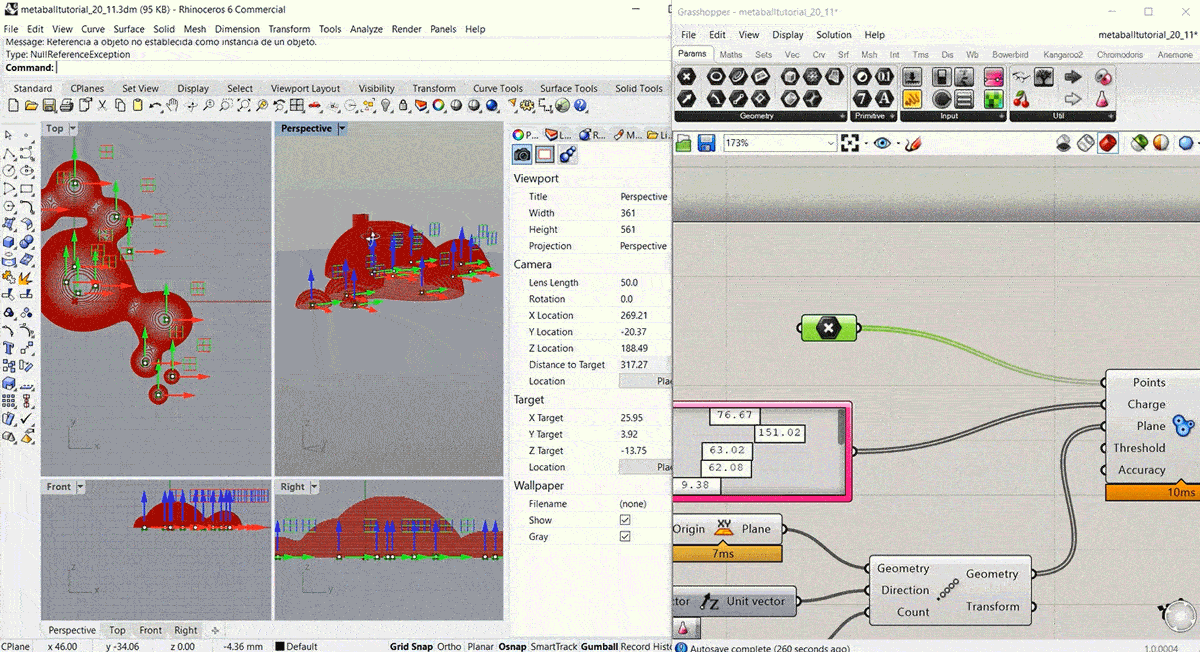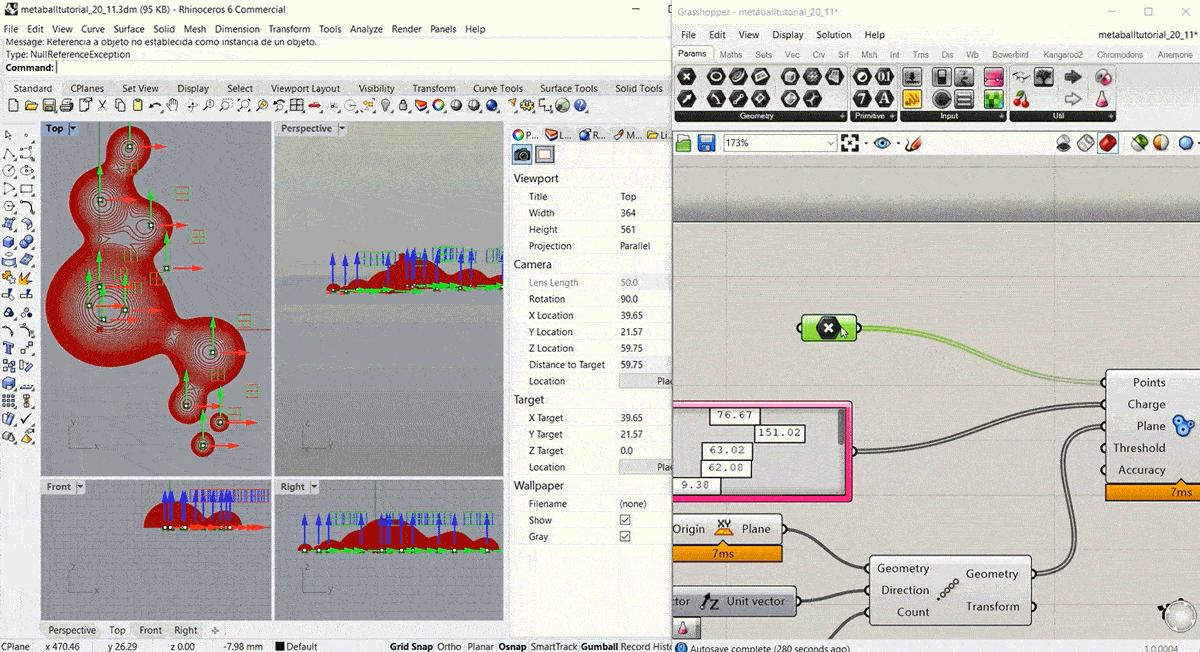
Step2: Fabricating the mould¶
Although we were supposed to use CNC milling machine for making the mould for this assignment, unfortunately ours is out of service, so I had to adapt the model to be fabricated using the laser cutter

Step3: Vacuum forming¶
First tests with the thermoforming machine


Moulding leather
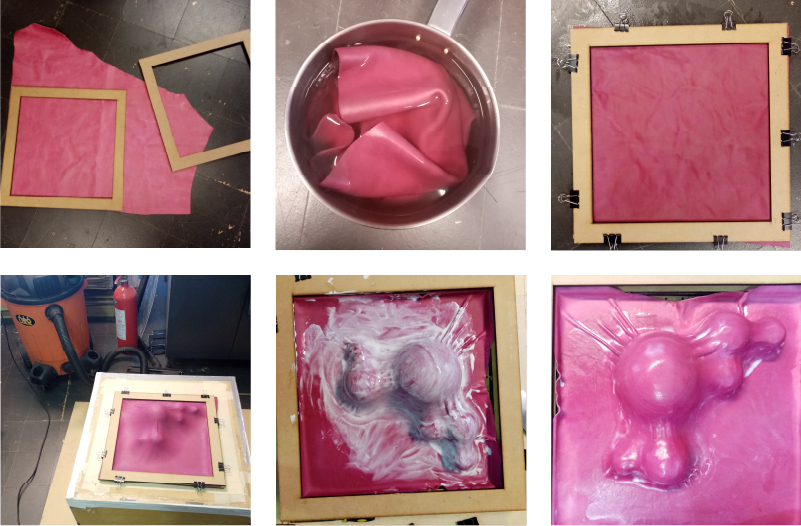
- Cut the leather to the size of the frame
- Place the leather in hot water, and let it rest for an hour
- Place the wet leather in the frame and tense it
- Place the mould in the center of the vacuum press, position the frame with the leather stretched upwards and turn on the vacuum
- Apply a layer of glue
- Let dry


Leather molding - Leather Sample 2¶
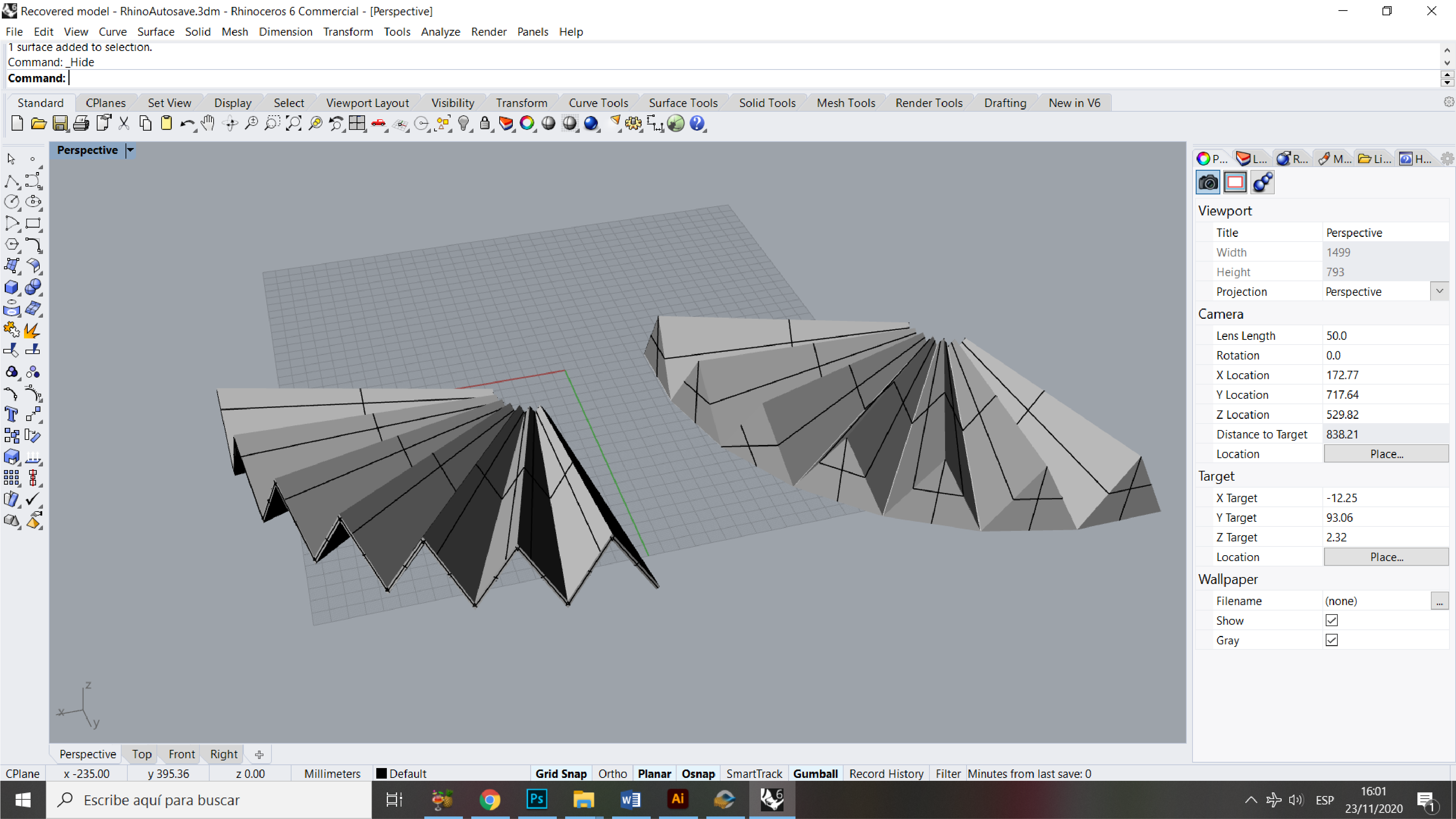
Step 1: Designing the mold¶
Rhino
Step2: Fabricating the mold¶
CuraUltimaker + 3Dprinting
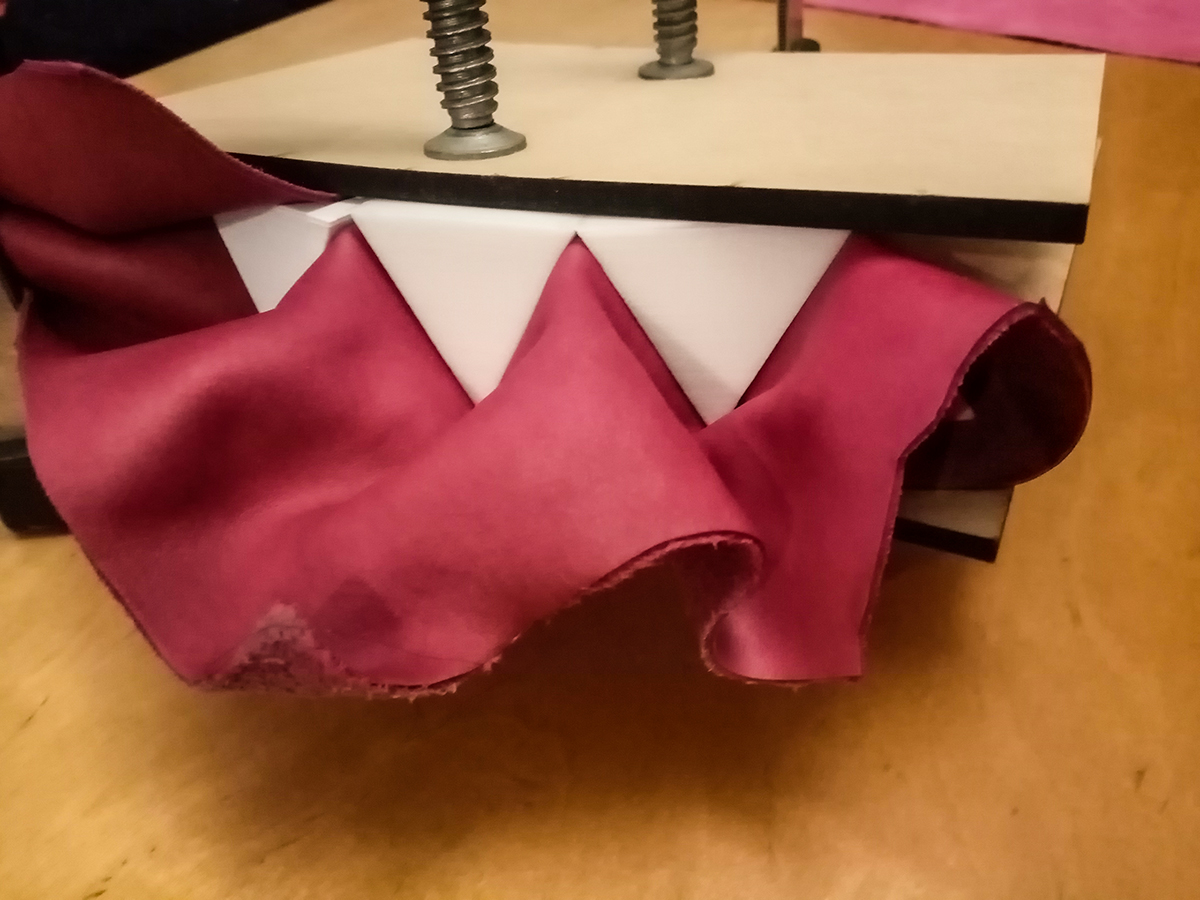
Step3: Wet molding¶
Preparing the leather + Preparing the molds + molding


Crystallization¶
"In chemistry, a crystal is matter that assumes a crystalline form, almost always a solid. The hallmark of this kind of structure is a repeating subunit of sorts, which is usually an atomic nucleus at the center of a geometric cube and ions carrying a different charge placed at the cube's corners or in the centers of its sides.
What Are Crystals? Lets review how chemists and physicists classify states of matter. A change in state of matter is not a change in the chemical composition of a substance (that is, its molecules don't change) but instead a change in the physical arrangement of the matter.
The three standard states of matter are, in order of increasing molecular kinetic energy, solid, liquid and gas.
When molecules are in the form of a solid, that means that their molecules have lower total and average kinetic energy (KE) than the same amount of that substance do in the liquid state, which in turn features a lower KE than the gaseous state for that substance.
Often, molecules in the form of a solid, the nuclei of which have virtually no freedom to move about in relation to each other, form regular, repeating patterns called lattices.
While these small (conceptual, not actual) lattices span only a molecule or two, their properties extend largely to the "macro" world. Quartz, on inspection, is quite evidently a "regular" sort of rock, with eye-pleasing geometric angles and lines; other crystals, many of them synthetic, capture, reflect and refract light in visually appealing ways and are popular in jewelry, architecture and elsewhere.
What Is a Solution? When a solid with molecules that consist of bonded ions (charged atoms or molecules) is placed in a liquid, the bonds of the solid may be broken, and the constituent atoms or molecules of the solid substance may become evenly dispersed throughout the liquid. When this is the case, the result is called a solution; when water is the liquid, is is called an aqueous solution,
In this context, the liquid is a solvent, and the solid is a solute. The amount of a solute than can be dissolved in a given amount of water or other solvent is, as you should expect, finite; in many cases, the solubility of a given substance in a given solvent also depends on the temperature at which this chemical reaction is occurring.
In general, as temperature rises, solubility increases, and as temperature drops, solubility decreases. This means that for a given amount of solute, a solution may form at one temperature, but solid my be present at a lower temperature.
At the point at which no more solute can be dissolved in solution, the solution is called saturated, and conditions are in place for crystal formation to occur. If more solution is added (or, in some cases, if the solution is cooled), more solute accumulates as the solution is now supersaturated. Crystals now begin to form as a result of favorable collisions between solute molecules in the ever-more-crowded solution." 1
Growing crystals process:
To make the crystals, I followed class notes seen in global presentation, but I also used Loes Bogers documentation, which is very complete and was a great help.
Ingredients¶
- Alum powder
- Water
- Textile swatch for the alum crystals to attach to (I used cotton fabric and cotton yarn)
Tools¶
- Cooker or kettle
- Glass jar
- Pan for boiling water (the glass jar has to fit inside the pan)
- Stirring spoon
- Wood stick
- Clips
- Yarn
Process¶
-
Preparation
Preparing the alum: Weigh 125g of Alum Preparing the textile swatch: Attach your fabric to the wood stick using yarn and clips. Place the glass jar inside the pan, and then place both in the cooker. Fill the pan with water (as much as you can without making the glass jar placed in the middle float or fall) and boil it. The preparation of the crystals will take place inside the glass jar, in a bain-marie, that will help keeping the crystal solution warm and cooling down very slow. In another pan, boil some more water Once the water is boiling, take both the pan and the jar and place them in a warm place. -
Dissolving the alum:
Measure 400 ml of boiling water and put it inside the glass jar (which is still inside the pan). Add the 125g of Alum, spoon by spoon, while stirring. This is a very important part. You have to pour alum until the solution is saturated, until no more alum dissolves. Try to avoid adding to much alum. When the saturated solution is ready, place your textile swatch inside the glass jar. Make sure its not folded, and that it doesn't touch the bottom or the glass jar. -
Leave the crystals to grow in a place without anyone moving or touching it, for 24hs. Some crystals might be formed after 8 hours.
-
Rinse them under cold tap water and let them dry.

Alum Crystal Samples





EXTRA: Textile composite¶
Textile Solidification sample
Step 1: Designing wood frame in Rhino
Step2: Fabricating the wood frame Corel + Laser cut in MDF 3mm + Assembling
Step3: Making the composite Preparing the textile + fabric tensioning + solidification with glue


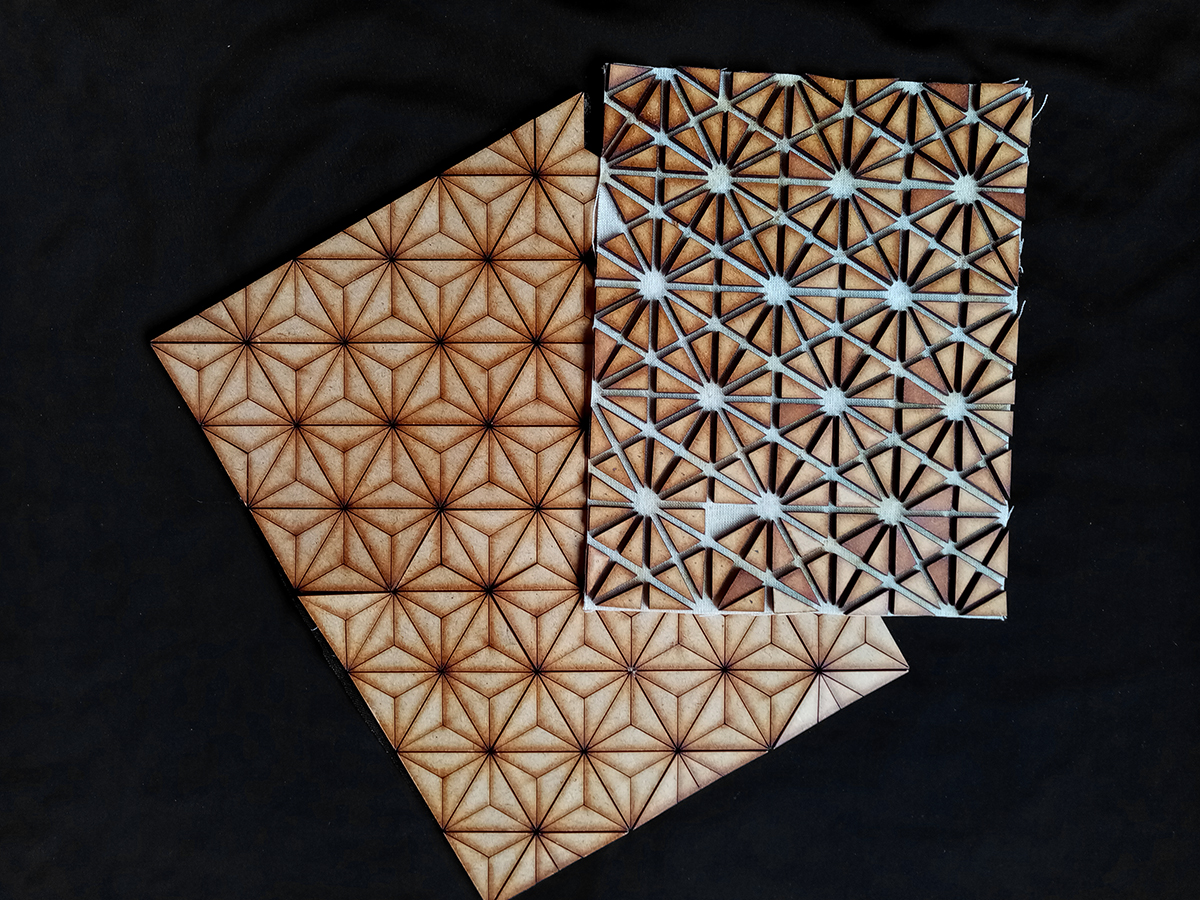
EXTRA: Wood+Textile Composite¶
Step 1: Designing wood patterns using Rhino
Step2: Laser cut the pieces in MDF 3mm
Step3: Making the composite Preparing textile swatches (cotton fabric) + attaching wood pieces to the fabric using glue



Files¶
All the files made for this assignment can be downloaded here.