03 BC living ink
Bacterial cellulose Bacterial cellulose is a highly pure form of cellulose that is produced by certain types of bacteria. It is known for its high tensile strength and high modulus, which makes it a good candidate for use in medical implants, textiles, and other applications that require strong and stiff materials.
I identified 3 different main problems to overcome trying to grow BC as a "living-ink"
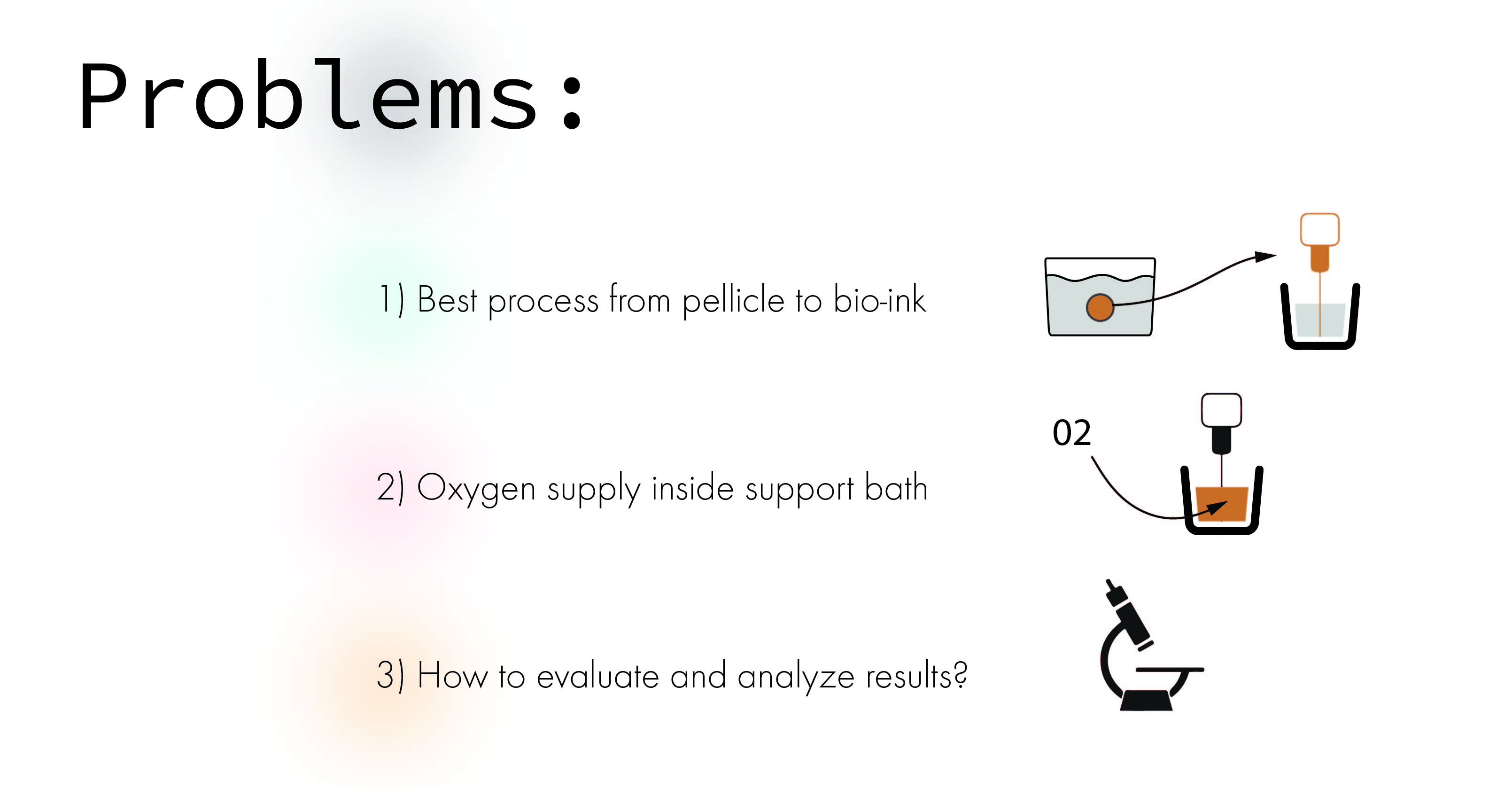
Problem 1: Pellicle to bio-ink¶
The bacteria and yeast in the SCOBY secrete a sticky, slimy substance called extracellular polymeric substances (EPS), which helps them adhere to surfaces and form a biofilm. This biofilm is a complex and highly organized structure composed of layers of microorganisms, EPS, and other organic and inorganic materials growing on the surface of the culture media where it is in contact with air.
The EPS produced by the SCOBY serves several important functions in the formation of the biofilm. It helps to anchor the microorganisms to the surface of the tea, provides a protective barrier against environmental stresses such as changes in temperature and pH, and facilitates communication and cooperation between different species of bacteria and yeast within the biofilm.
Overall, the formation of a SCOBY biofilm is a complex and dynamic process that involves the symbiotic interactions between multiple species of microorganisms and their environment.
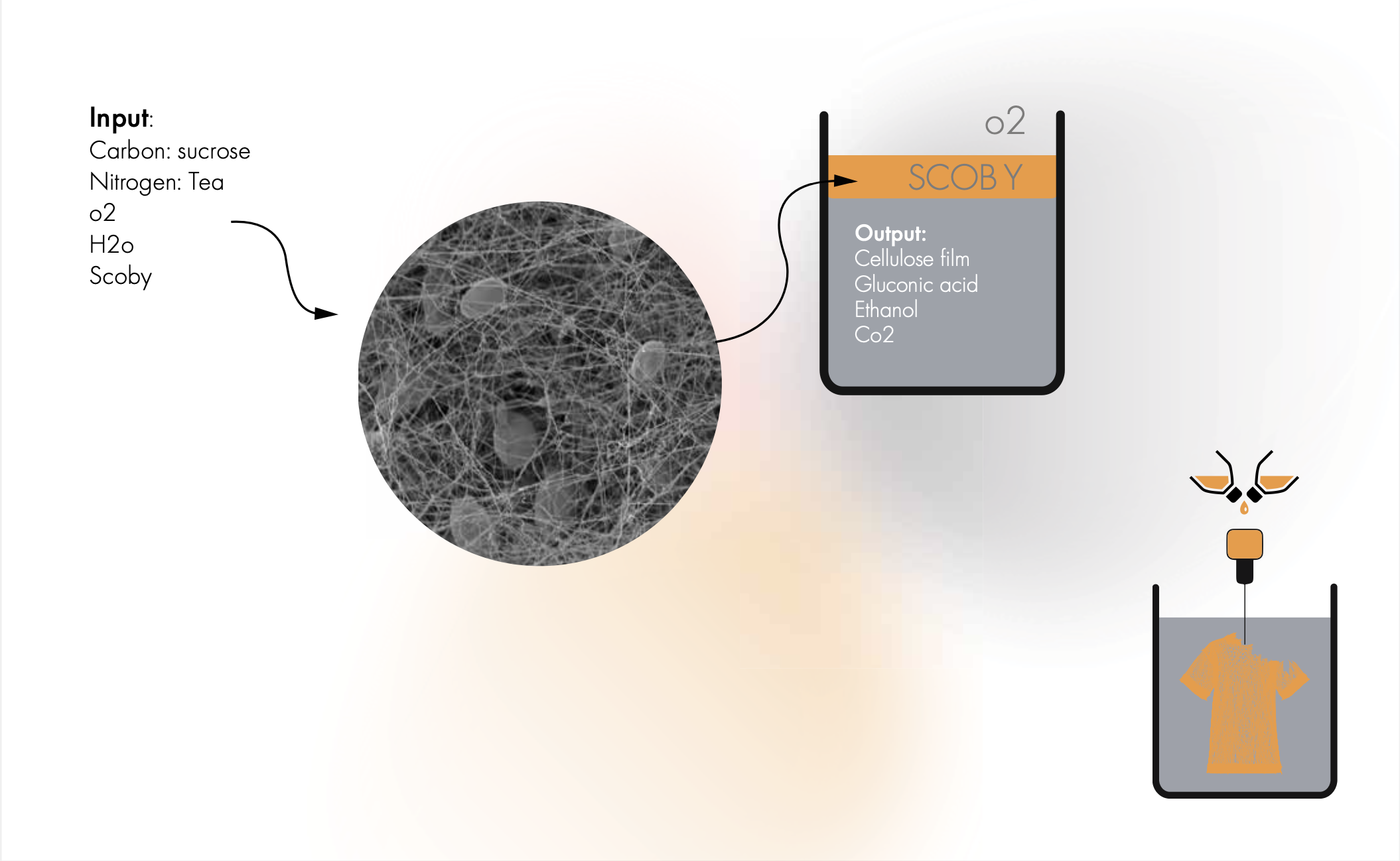
If we wanna print living BC we need to find a way of guiding the cells to grow in a completly new way that it would not naturally do.
This race a few different challenges:
br />
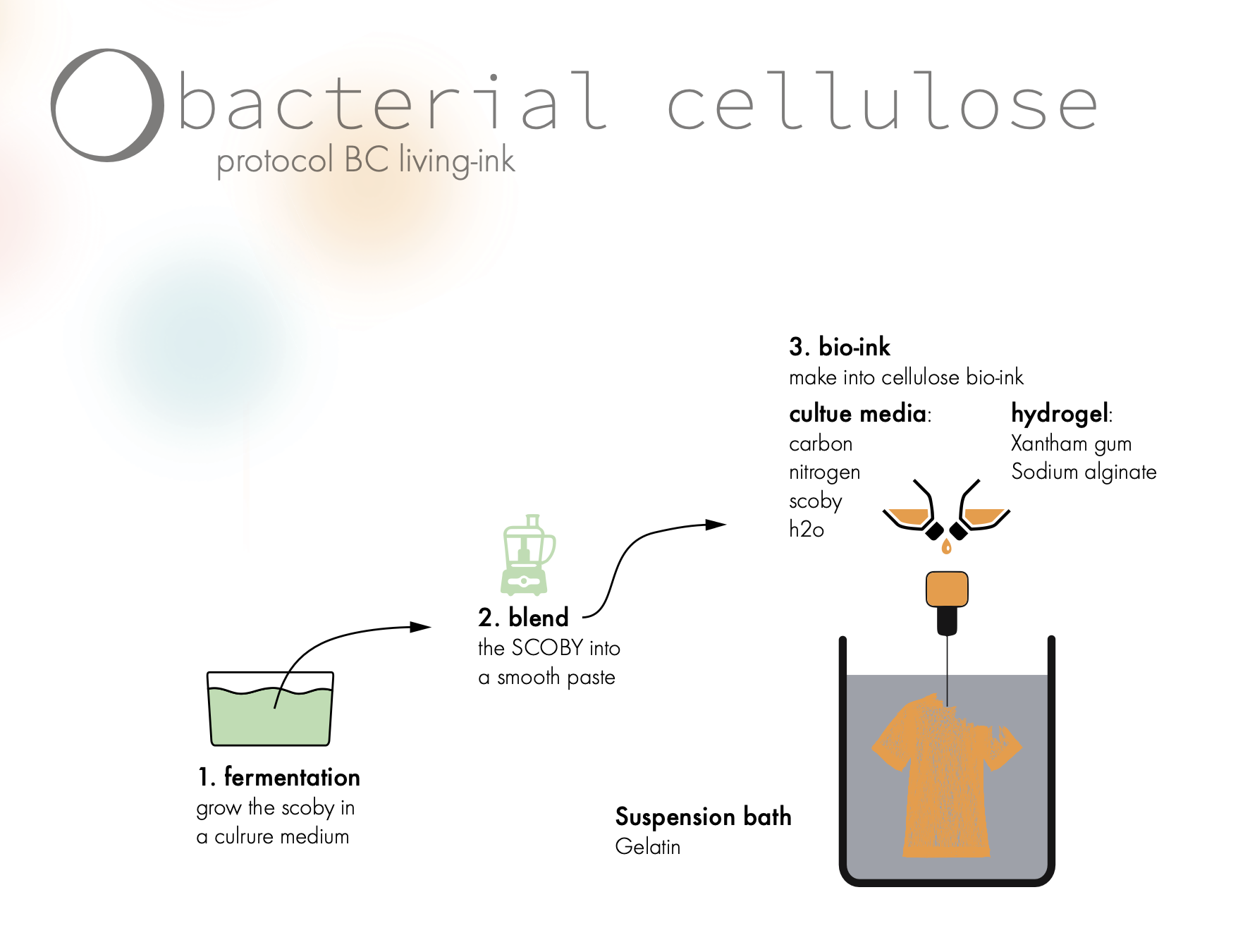
When fermenting the BC culture it will form a SCOBY, but what is the best way to extract the BC into a culture smooth enough that it is suitable for printing? br />br />
Here is 3 different roadmaps outlined:
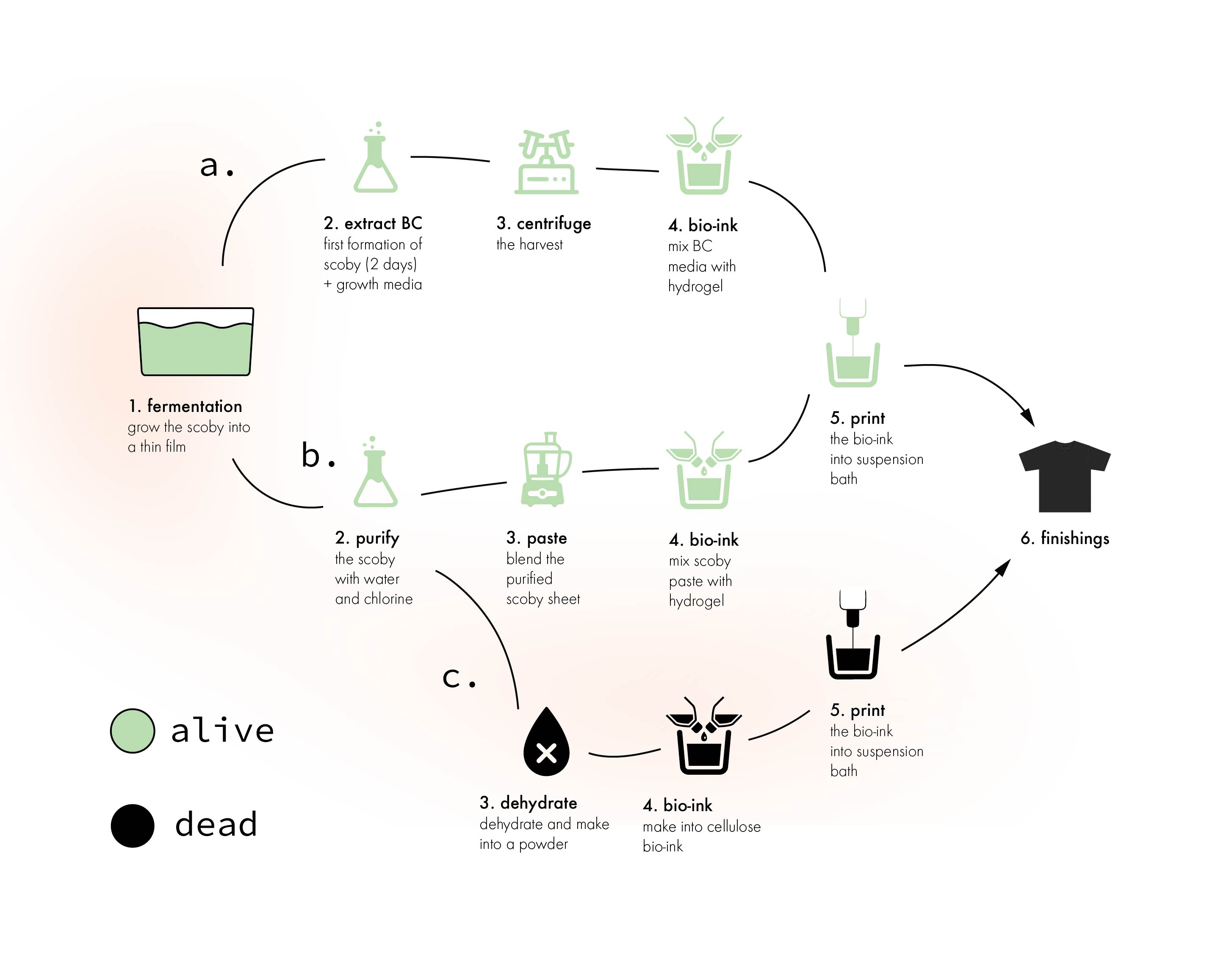
roadmap p1A¶
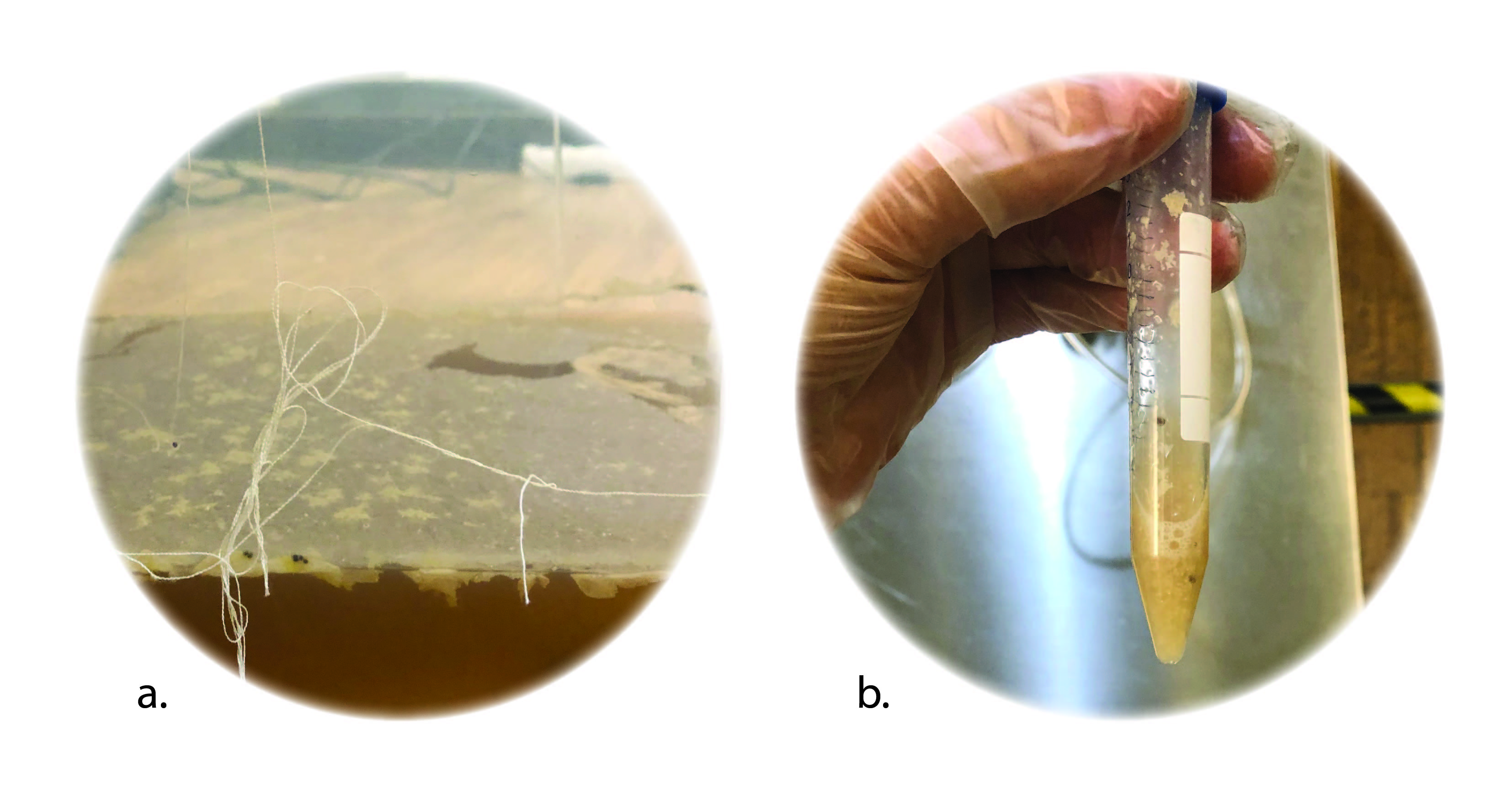 a thin layer of Scoby film+ growth media extracted from the fermentation bath
a thin layer of Scoby film+ growth media extracted from the fermentation bath
 left: BC bio-ink right: after 2 days
left: BC bio-ink right: after 2 days
* 2 gr Sodium Alginate
* 100 ml water
* 3ml BC solution
* magnetic stirrer
* glas jars
* Sodium alginate combined with the BC culture
* dissolved and vortexed at 30°C
* Once cooled, the hydrogel was placed in a syringe with a 1inch 27G needle for printing
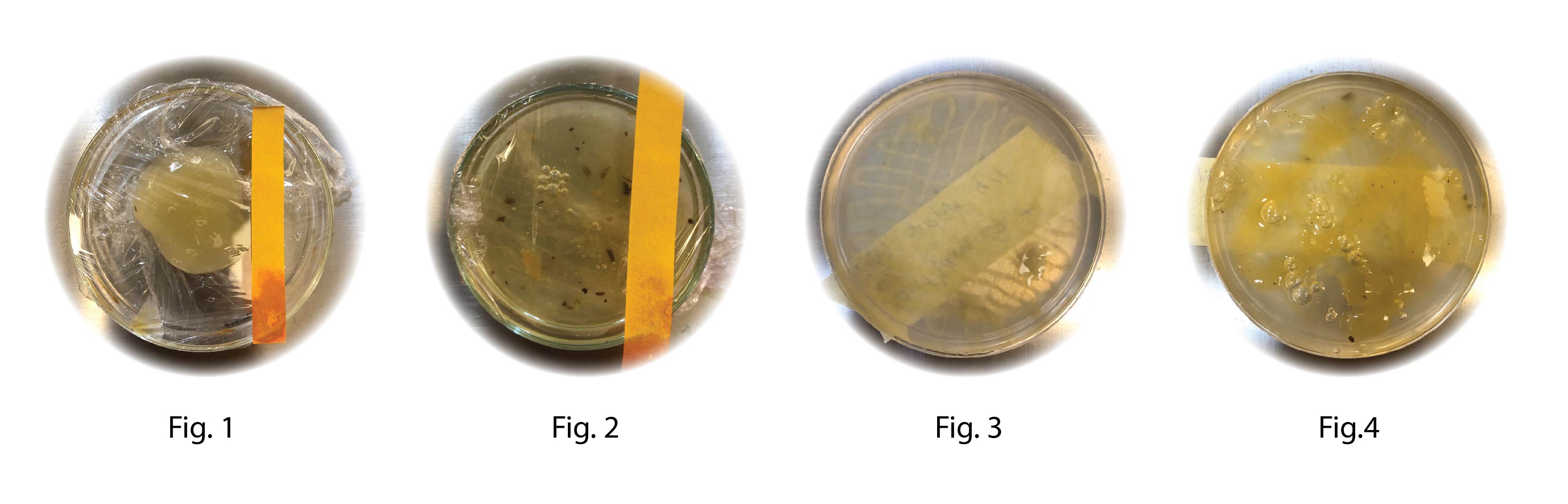 The Kombucha bath was extruded after 8 days of growth and mixed with two different polymers using a magnetic stirrer . it was left for an incubation period of 24 hours at 30°C before printing directly into a petridish (Fig.1 and Fig 2.) as well as into SB-b (Fig. 3 and Fig. 4)
The Kombucha bath was extruded after 8 days of growth and mixed with two different polymers using a magnetic stirrer . it was left for an incubation period of 24 hours at 30°C before printing directly into a petridish (Fig.1 and Fig 2.) as well as into SB-b (Fig. 3 and Fig. 4)
Fig 2 Reciepe: BC 2
•2% sodium alginate
•Kombucha recipe B
•crosslinked with 1% Calcium Chloride
Nozzle: 1.5 mm plastic
Pressure: 10 bar
Fig. 1 Recipe: BC 3
•1% xanthan gum
•Kombucha recipe B
•crosslinked with Citric Acid
Nozzle: 0.8 mm brown
Pressure: 10 bar
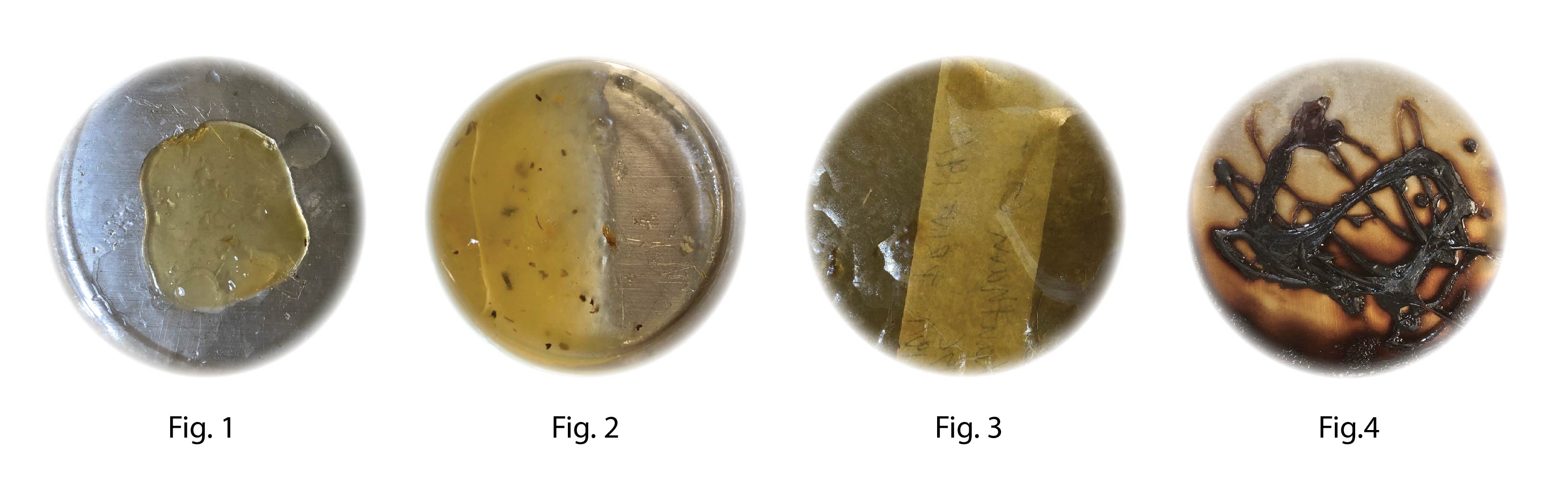 After 7 days incubation at 30°C Xantham gum sample (Fig1) had become hard slighly bendable and sticky. Fig 2 Alginate reciepe still soft. Fig 3 and 4 completly dried out
After 7 days incubation at 30°C Xantham gum sample (Fig1) had become hard slighly bendable and sticky. Fig 2 Alginate reciepe still soft. Fig 3 and 4 completly dried out
Conclusion: The samples was drying out to fast even though they where placed in an incubation chamber with high humidy, no BC growth was visible.
It is important to find a methology to detect growth for the future I will take a microscopic image before and after incubation in order to better detect growth.
The alginate sample (Fig 2) is the only one that remained soft which might indicate that the crosslinking with Calcium Chloride might create an outer shelf that prevents the water from evaporate however it might also prevent BC to grow.
Recipe BC5:
To investigate how much the viscosity of the hydrogel is effecting the growth of the BC pure growth medium (extracted from Kombucha reciepe C) was printed into the support bath (SB-2)
Conclusion: No clear sign of growth
roadmap p1B¶
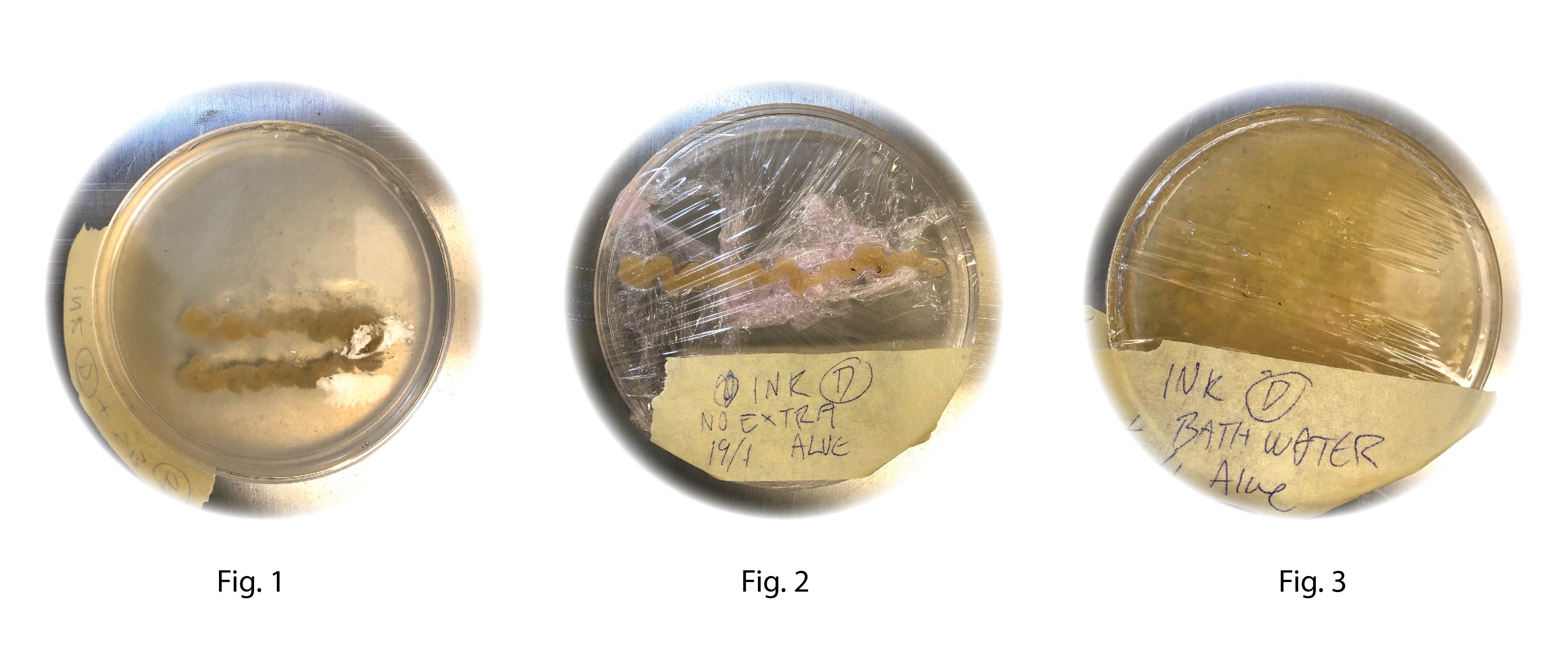 A piece of the SCOBY mother was blended together with equal parts mother medium and Kombucha recipe C. using a food processor. Fig 1 printed into SB-2, Fig. 3 printed into the Kombucha water. All samples was covered with a plastic film (with small airholes) and incubated at 30°C
A piece of the SCOBY mother was blended together with equal parts mother medium and Kombucha recipe C. using a food processor. Fig 1 printed into SB-2, Fig. 3 printed into the Kombucha water. All samples was covered with a plastic film (with small airholes) and incubated at 30°C
Recipe BC4:
Fig1. Printed into SB-B Fig.2 Printed straight into Petri dish Fig.3 printed into Kombucha reciepe C. All the samples were left for incubation at 30°C
• Scoby
• 10 ml mother medium
• 10 ml Kombucha recipe C
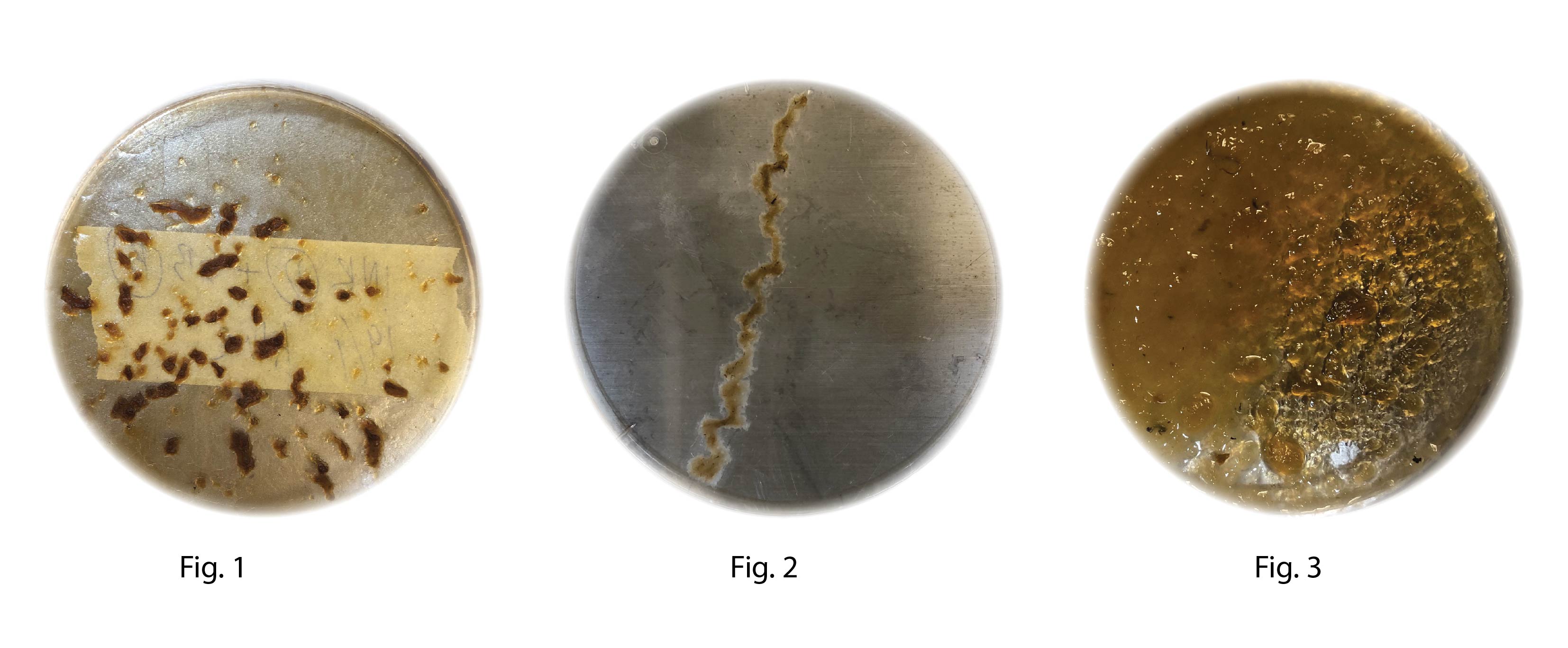
Fig 1 and 2 dried out after only 3 days Fig 3 is still sticky after 7 days of growth
Conclusion: No clear sign of growth
Recipe BC4:
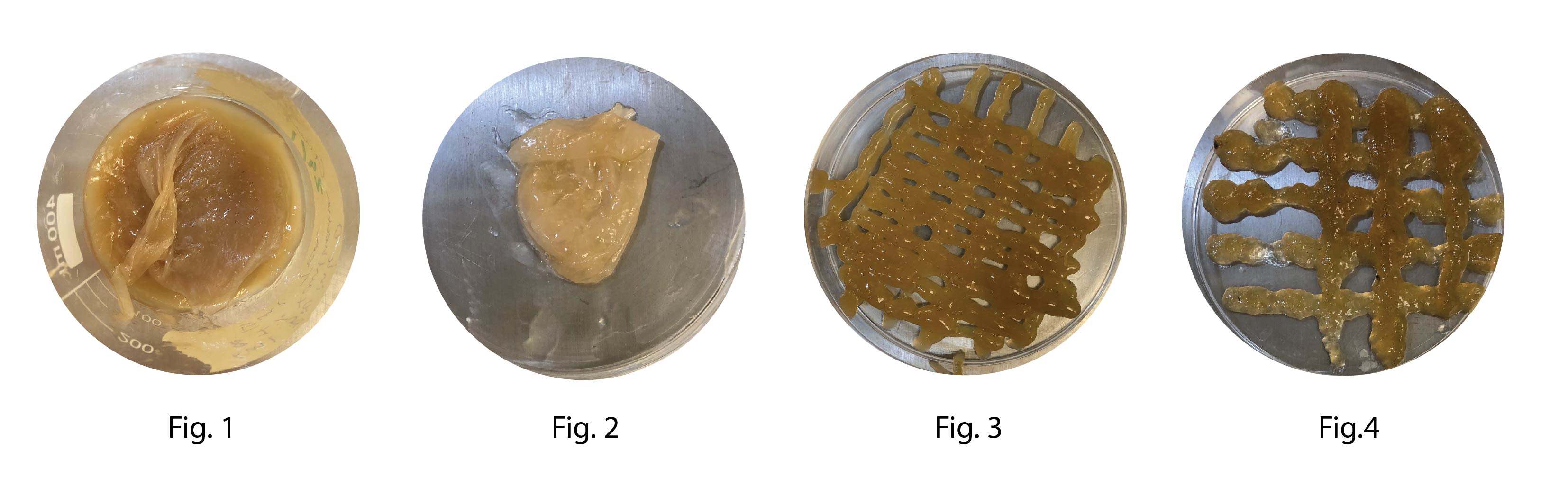 100 ml BC4 was mixed with 7 ml kombucha growth medium (reciepe C) and divided into to badges.
Fig.3 1% Xantham gum
100 ml BC4 was mixed with 7 ml kombucha growth medium (reciepe C) and divided into to badges.
Fig.3 1% Xantham gum
Fig.4 2% Sodium Alginate
Fig 1 after 3 days of growth at 30°C a thin film had form on top of the petridish it is unclear if this is the Xantham gum that dried out or BC growth. The film is drying to further investigate the result (Fig 2)
Problem 2: Oxygen supply¶
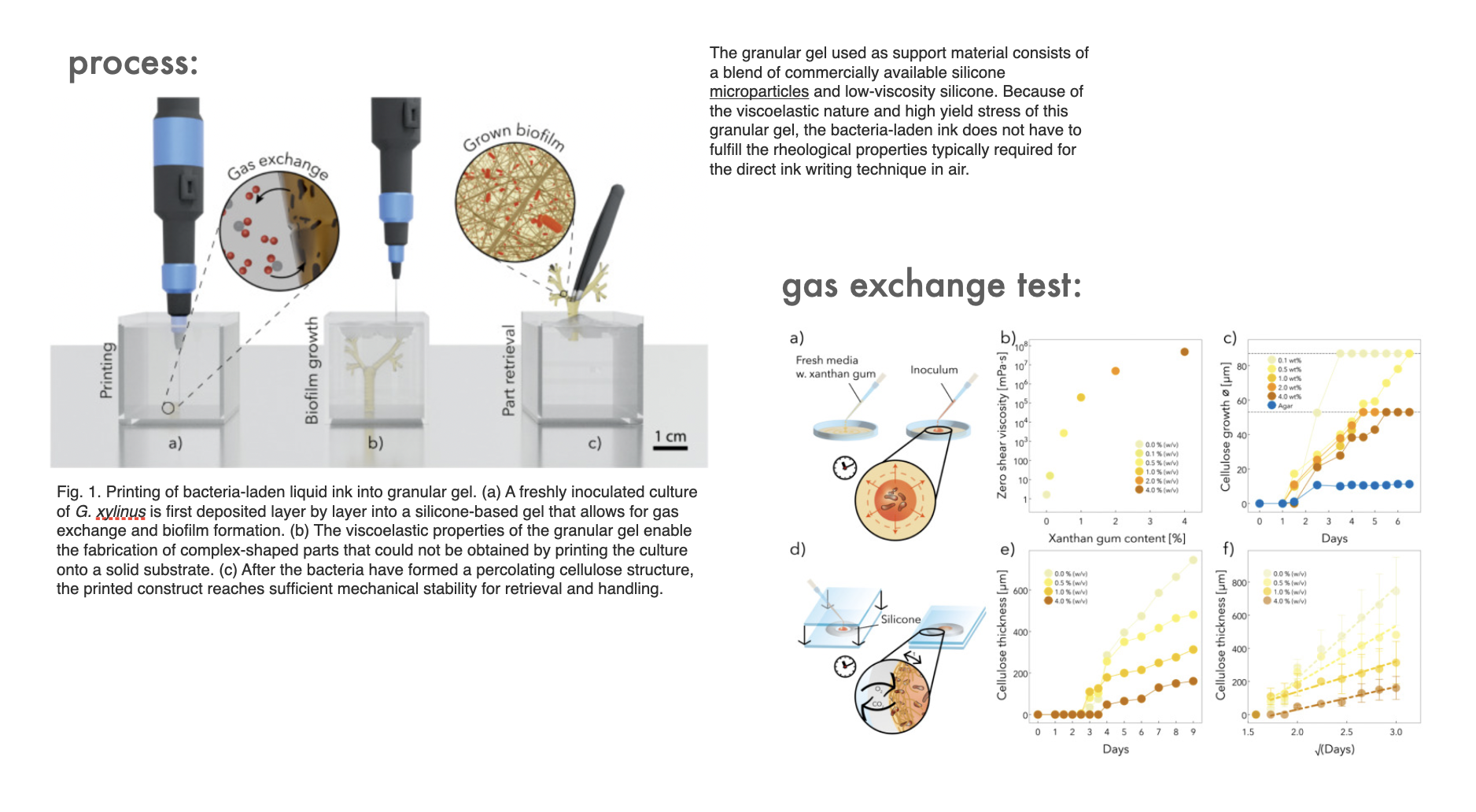
One of the bigger problems around printing living cells into a support bath is the oxygen supply that all living organism needs. This is a study outlining a gas exchange that supply BC with enough supply that it can form a network
However, it is worth noting that the BC is not growing as well in the gel as it would with an unlimited supply of air
"Microscopic images reveal that bacteria embedded in the granular gel lead to a more heterogeneous cellulose network with a lower fiber density compared to biofilms exposed to air. Indeed, the density of cellulose fibers in the ink decreases from 5.23 to 1.65 vol%".
So how could we solve this issue, here is five different possible ideas for how to solve this issue:
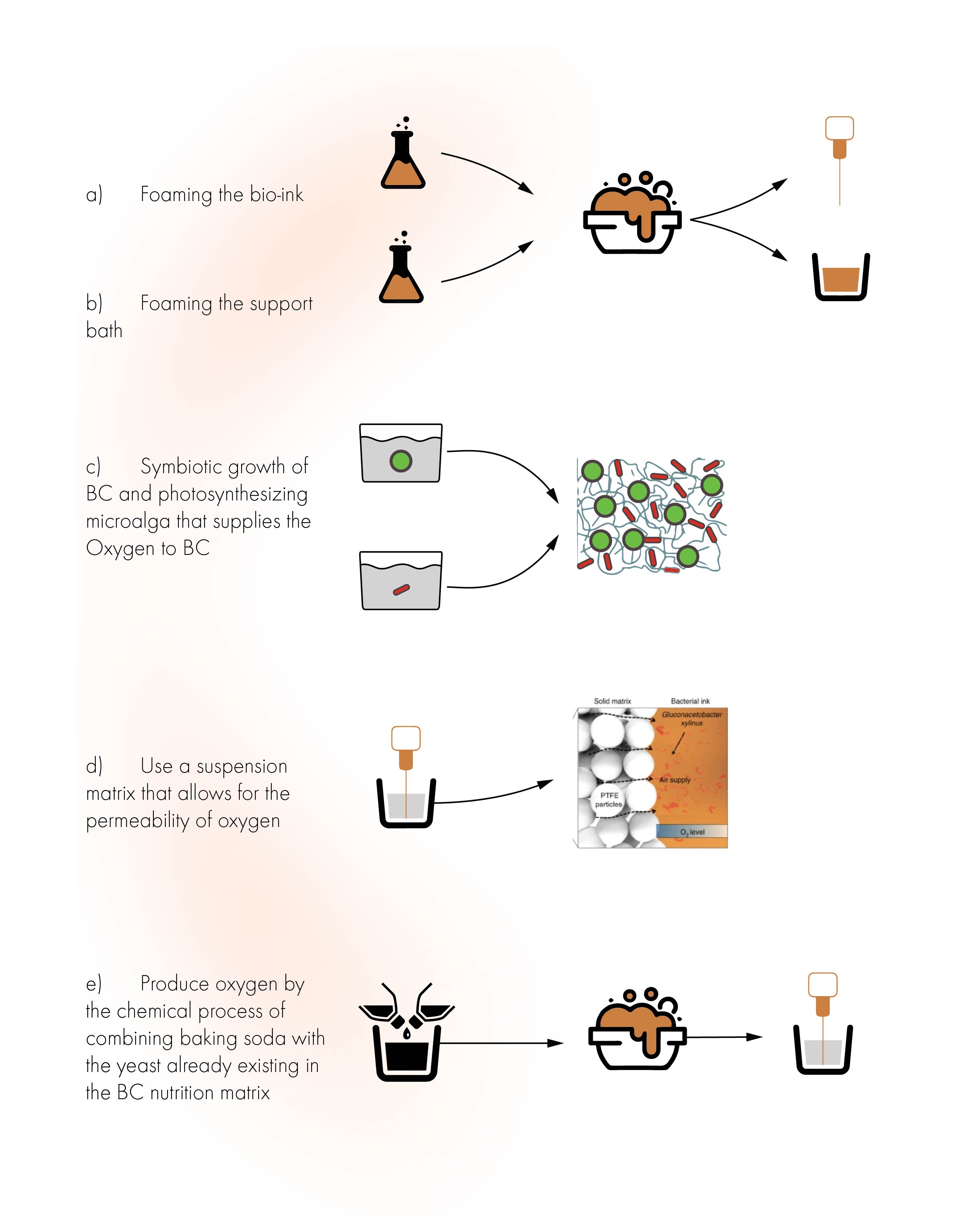 5 different possible solutions for the oxygen supply problem
5 different possible solutions for the oxygen supply problem
Experiment 1: roadmap P2 A+ b¶
* 2% Sodium Alginate
* 2% CMC
* 5% Glycerin
* water
* 1 tbs ink
* 1% CMC
* 5% Glycerin
* water
* 1 tbs ink
* magnetic stirrer
* glas jars
* Polymer combined with the food colorand
* dissolved and vortexed at 60o C
* Once cooled, the hydrogel was placed in a syringe for printing
Print setting: B4
Nozzle: 0.8 mm brown
Pressure: 12 bar
Print setting: C1
Nozzle: brown
Pressure: 12 bar
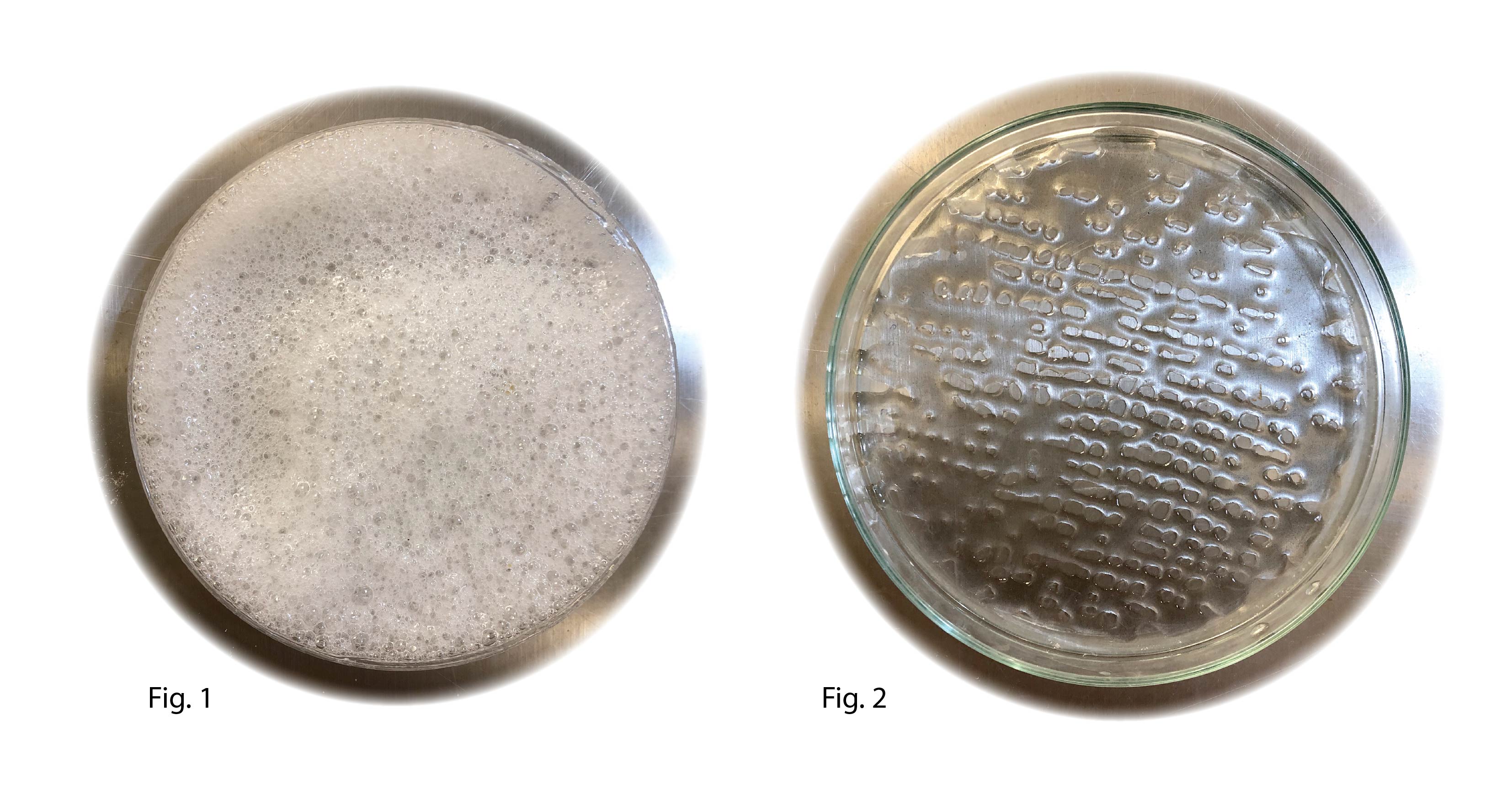 support bath: SB-b was blended for 1 min to create a foam (Fig1) before placing it in the fridge (6°C) for 12 hour. For the printing bio-ink B4 and C4 was used
support bath: SB-b was blended for 1 min to create a foam (Fig1) before placing it in the fridge (6°C) for 12 hour. For the printing bio-ink B4 and C4 was used
 Bio-ink: 2% of soap was blended into recipes B4 and C4 using an electric mixer (Fig1). Fig2 the foamed bio-inks was immediately printed into petri-dishes B4 was crosslinked with Calcium Chloride and C1 dried at 40°C for 12h. Fig3 was printed into SB-B
Bio-ink: 2% of soap was blended into recipes B4 and C4 using an electric mixer (Fig1). Fig2 the foamed bio-inks was immediately printed into petri-dishes B4 was crosslinked with Calcium Chloride and C1 dried at 40°C for 12h. Fig3 was printed into SB-B
Conclusion: The foamed support bath was not dense enough to support the ink that fell to the bottom of the petri dish, therefore this does not appear to be a good solution.
The experiments with the foamed bio-inks appeared to be more successful, the bubbles sustained the force from the extrusion and the density of the support bath.
However, the bubbles do affect the smoothness and the look of the print.
It is also worth considering if the soap will affect the growth of BC.
The next step would be to repeat the bio-ink foaming process using living-ink.
roadmap p2c¶
The idea is supported by two independent studies that report of successful insitu Symbiotic growth of BC and photosynthesizing microalga that through photosynthesis supplies the required Oxygen to BC
Study 1 BC strand: Acetobacter aceti NCIMB 8132 and microalgae Chlamydomonas reinhardtii cc-124)
Study 2 BC strand: Gluconacebacter xylinu and microalgae Chlamydomonas reinhardtii cc-124)
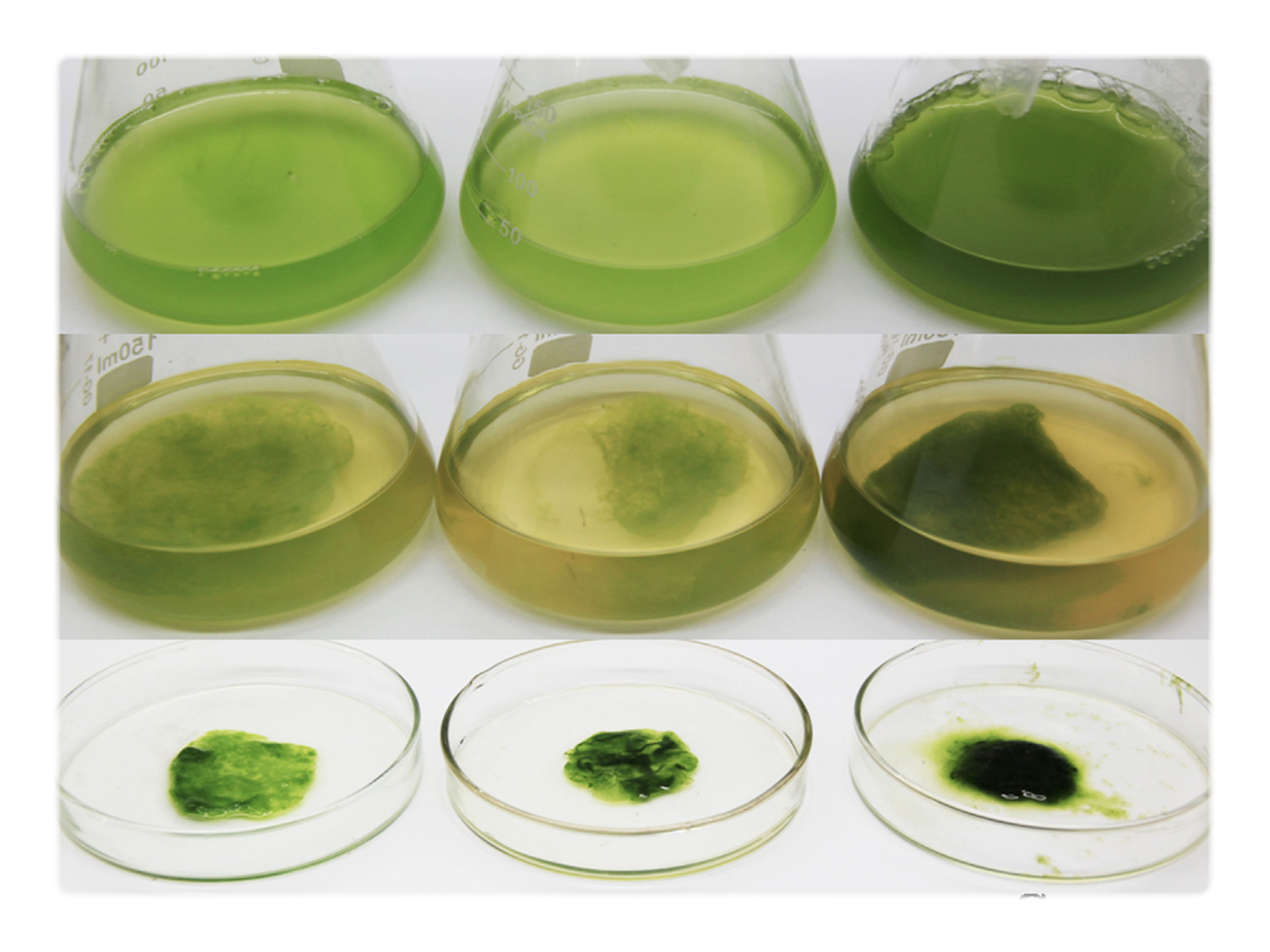
Since the oxygen are supplied from the algae the growth structure of BC completely change from a pellicle to 3D structure.
Conclusion: Algae strands was ordered and grown in a F2 media however the population was never dense enough that experiements was possible to conduct.
roadmap p2d¶
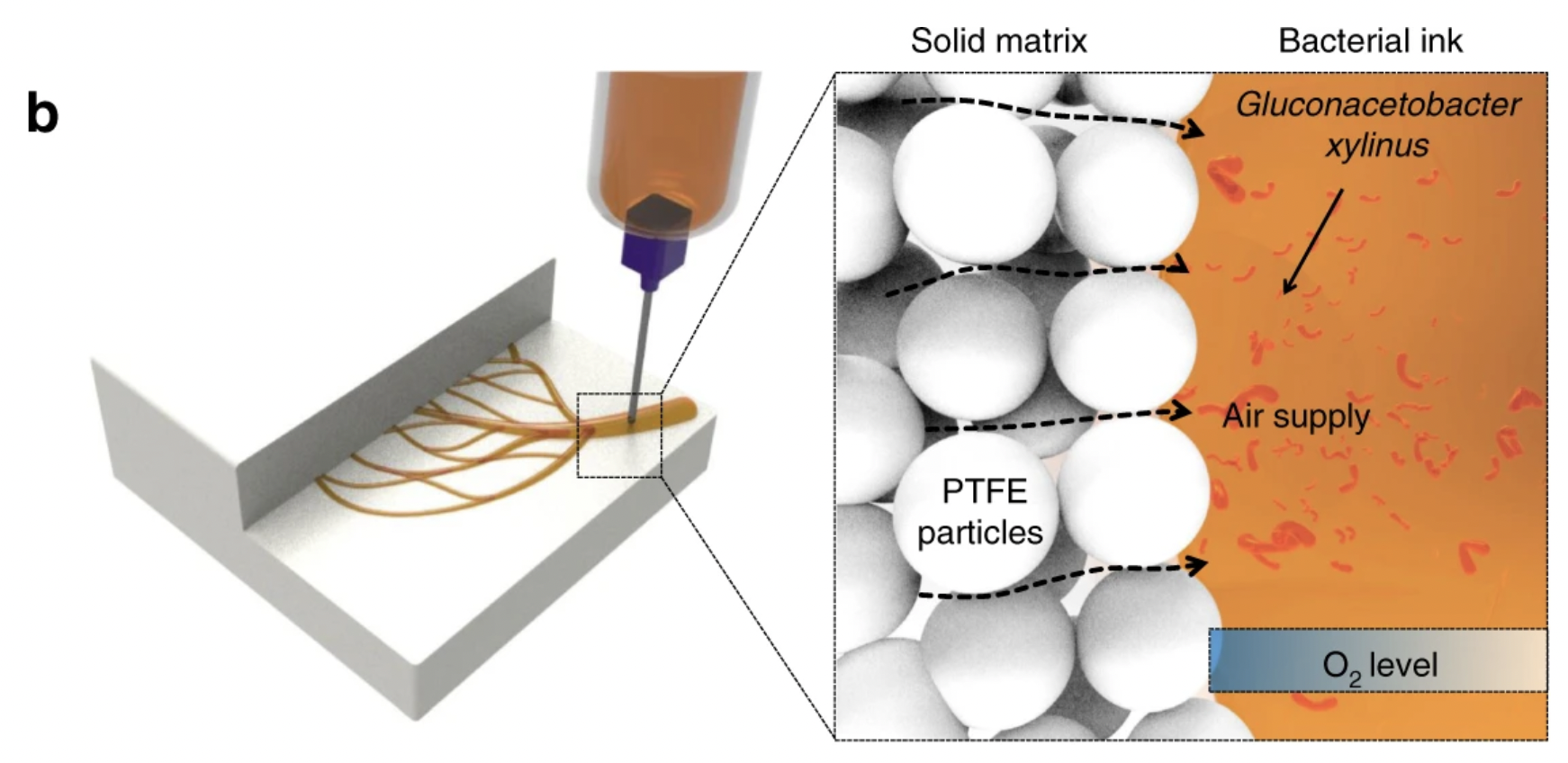
This is a study using a suspension matrix that allows for the permeability of oxygen. It is a powder-based suspension matrix of polytetrafluoroethylene (PTFE) microparticles and a hydrogel ink containing an incubation medium, bacteria (Gluconacebacter xylinus), and cellulose nanofibers (CNFs)
the process is called SMAP.
Conclusion: Due to the toxic nature of PTFE this solution was demead unsustainable and therefore not realised however it would be a interesting further research to look for other powders with similair properties as PTFE
roadmap p1e¶
 2H2O2 --> 2H2O + O2 . When yeast (already in SCOBY) come in contact with baking soda it produces oxygen, more info here
2H2O2 --> 2H2O + O2 . When yeast (already in SCOBY) come in contact with baking soda it produces oxygen, more info here
Kombucha "mother" medium was carefully injecteded into an airsealed bag with Baking soda. The reaction was immediate and the bag was expanding.
Conclusion: Even thogh the chemical reaction was succesful it is not ideal that reaction is immediate, we would rather want the oxygen production to start after printing. Therefor this might not be the best process for this project.
Experiment 2: BC samples:¶
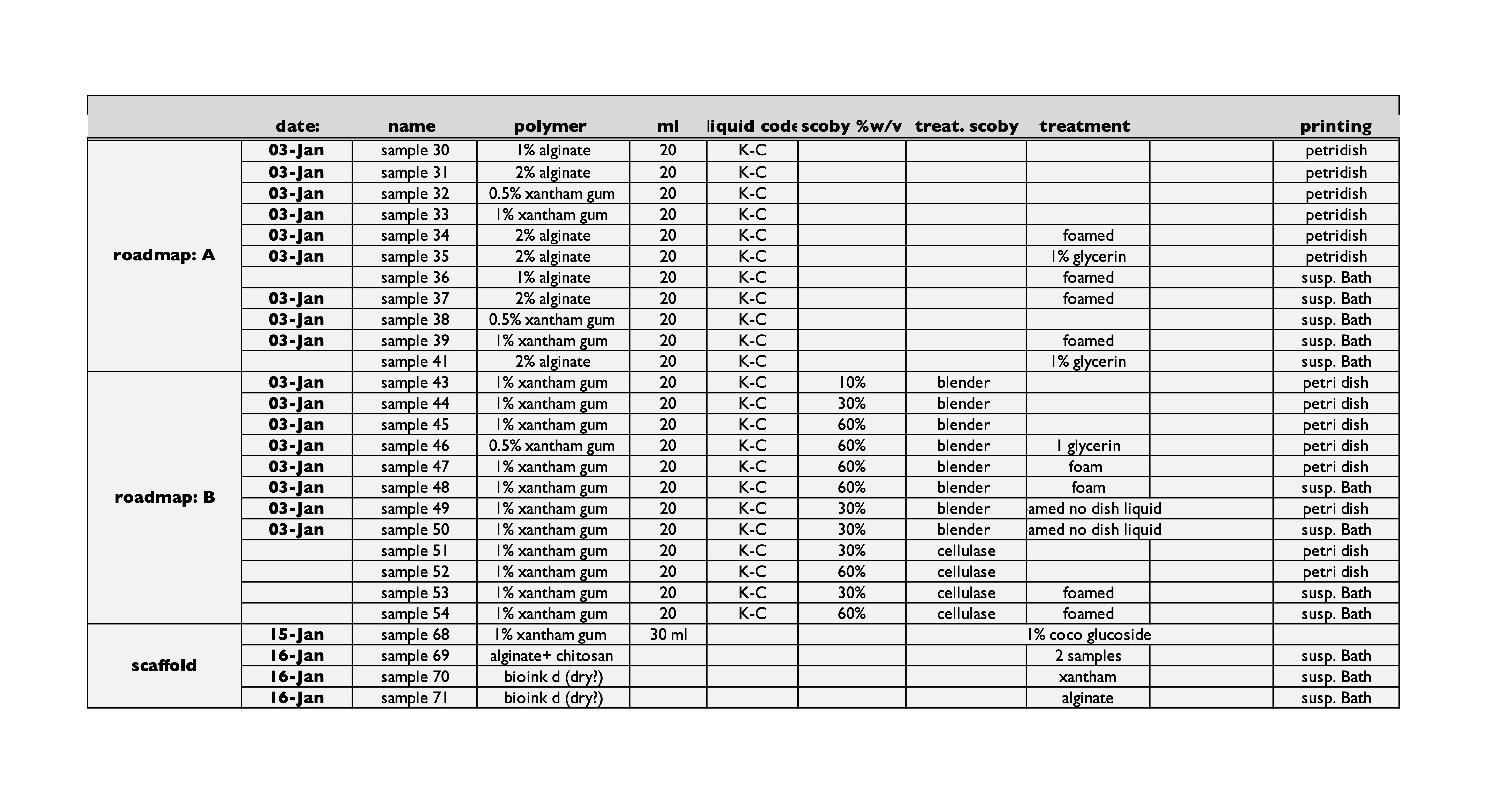
40 samples was launched (Sample 30-71) in Petri dishes as well as in small size plastic bottles containing 4% gelatin support baths. Additionally, 5 control samples was made for some of the samples in order to monitor the growth rate with an optical microscope.
See excel sheet for detailed breakdown of recipe’s.
All samples were left for 2 weeks of growth.
The control samples turned out not to be useful since our microscope wasn’t able to detect the growth. An SEM microscope is required.
Moreover most the samples didn't show enough growth that it was really possible to extract any reliably data from them. However here is a few thoughts from the results:
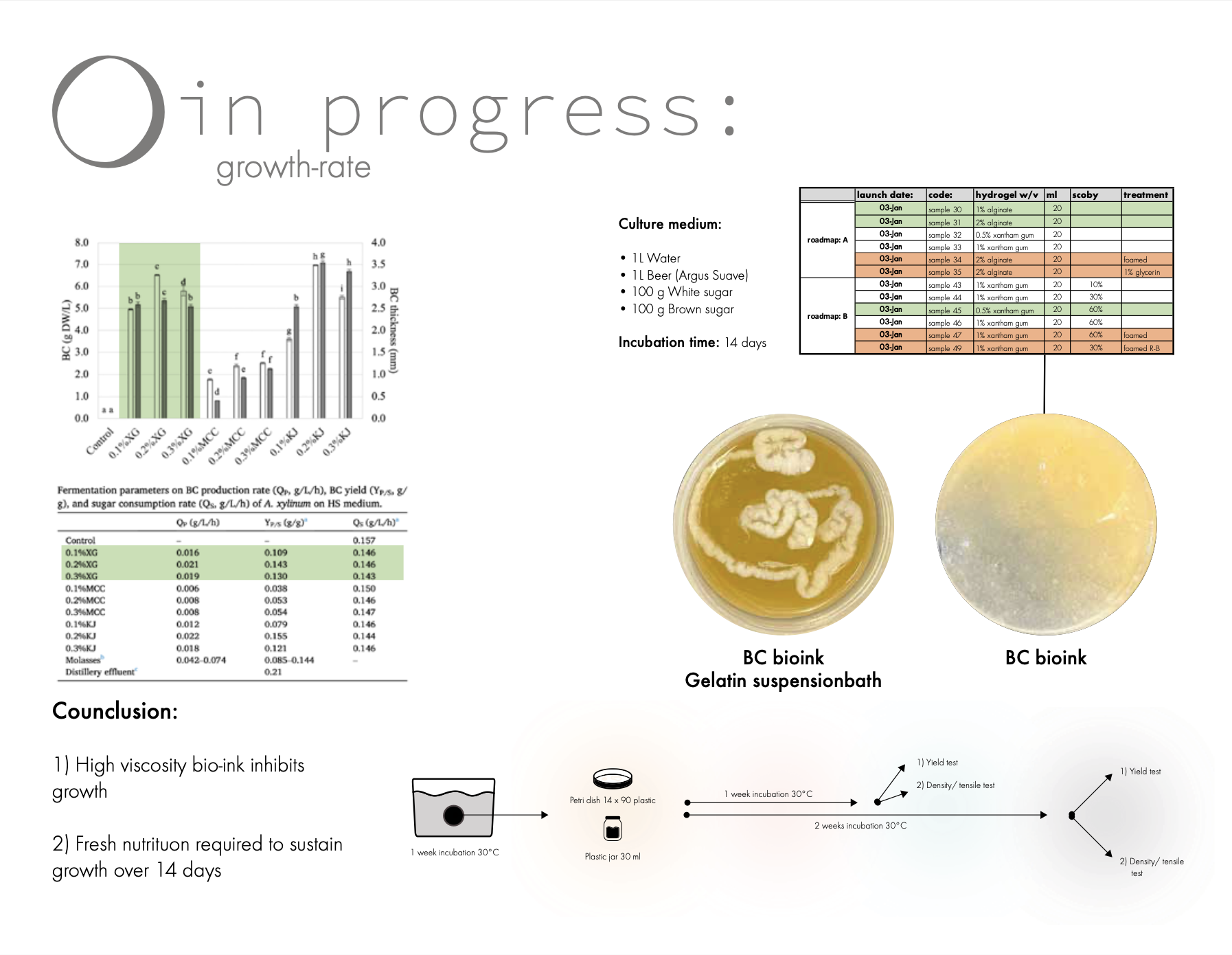
Conclusion
1) The sample with lowest viscosity (least amount of polymer) seem to grow the best this is supported by this study about growth rate for bacterial cellulose hydroids.
2) The growth rate seemed to stop after 7 days this might be because of lack of nutrition.
For future experiment I decided to either decrease the hydroid percentage (from 1-2% polymer to 0.2-0.5) or increase it significant 50-100% SCOBY since according to previous studies bacterial cellulose have a self-regenerative ability
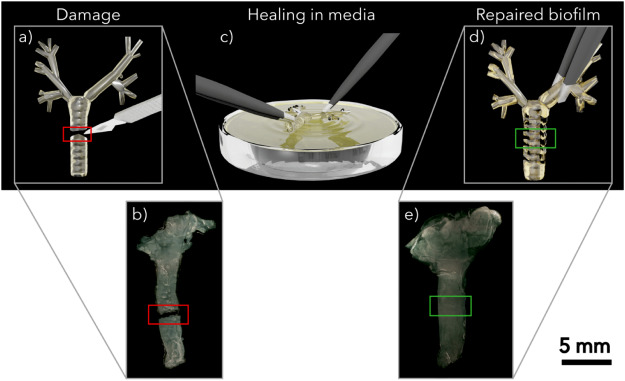
link
moreover, I will include fresh culture media to the recipe’s in order to boost the growth.
•10% w/v suger
•0.5% w/v yeast extract
•0.5% w/v instant coffee (nitrogen source)
Oxygen: Some foaming attempts have been made however they don't seem to be able to sustain it volume over longer time, based on this study I will use an icecream stabilizer for the foaming this will allow me to create a more stable and foam with smaller bubbles.
Based on this study I developed 4 new roadmaps for future experiments:
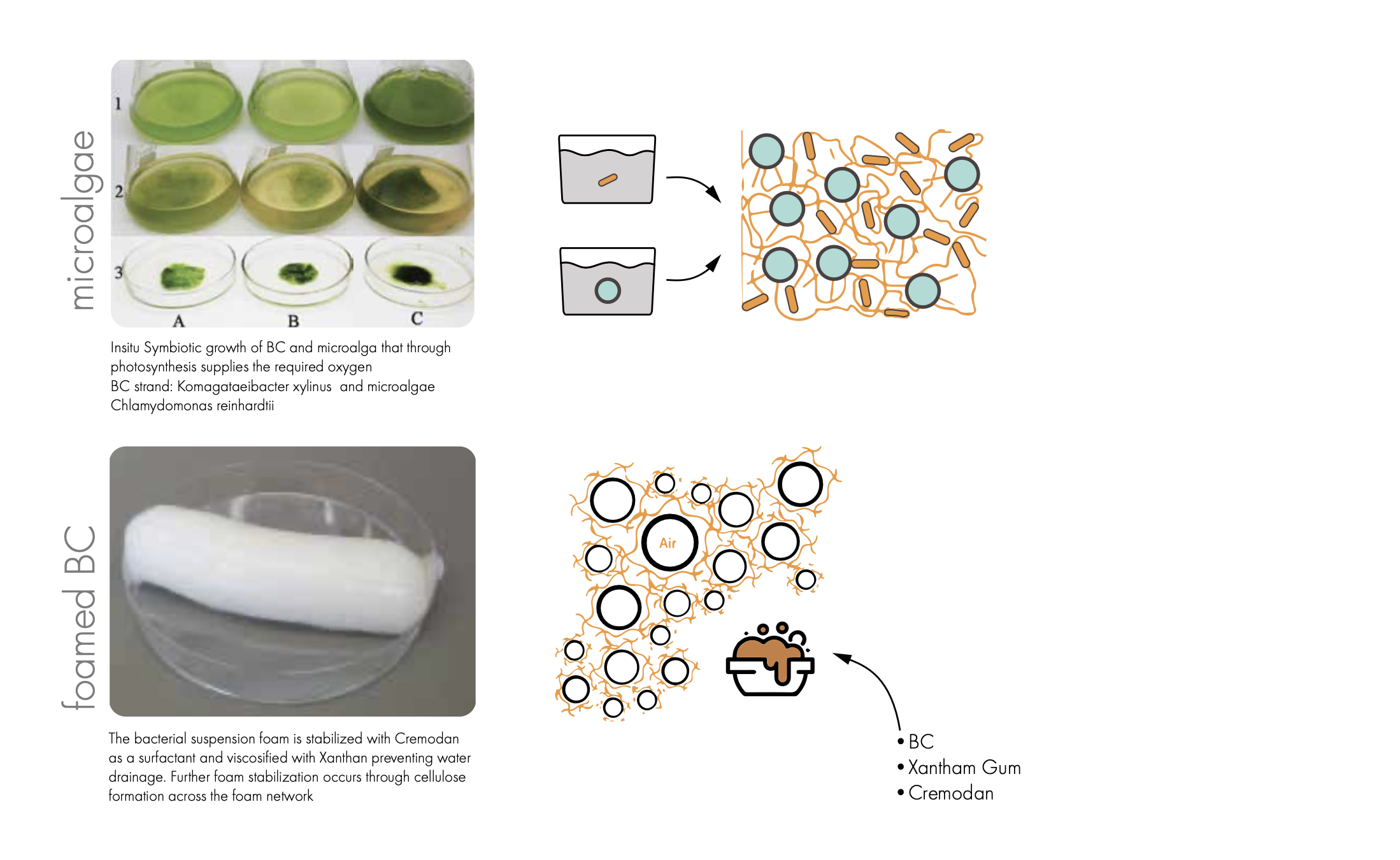
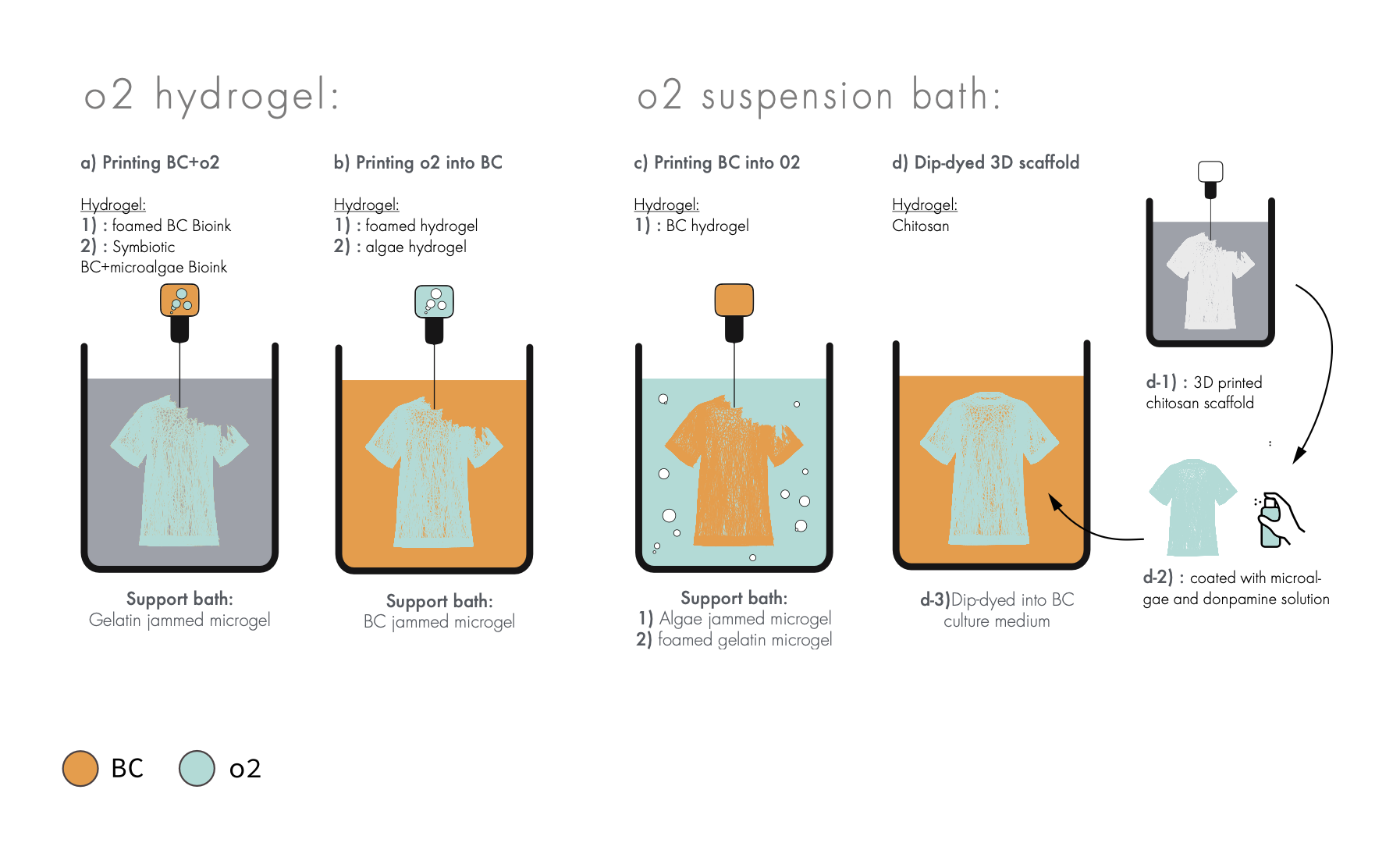
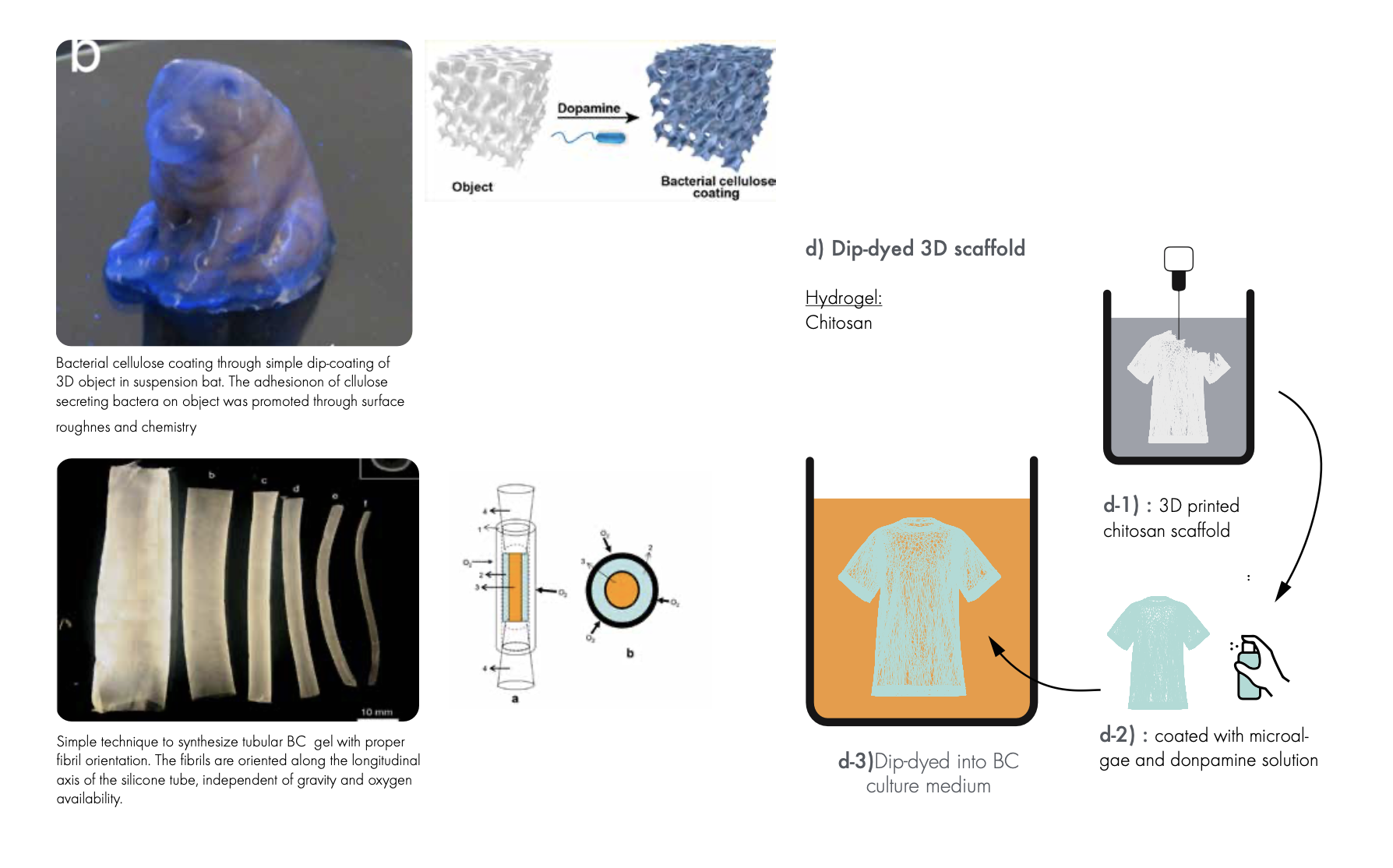 link
link
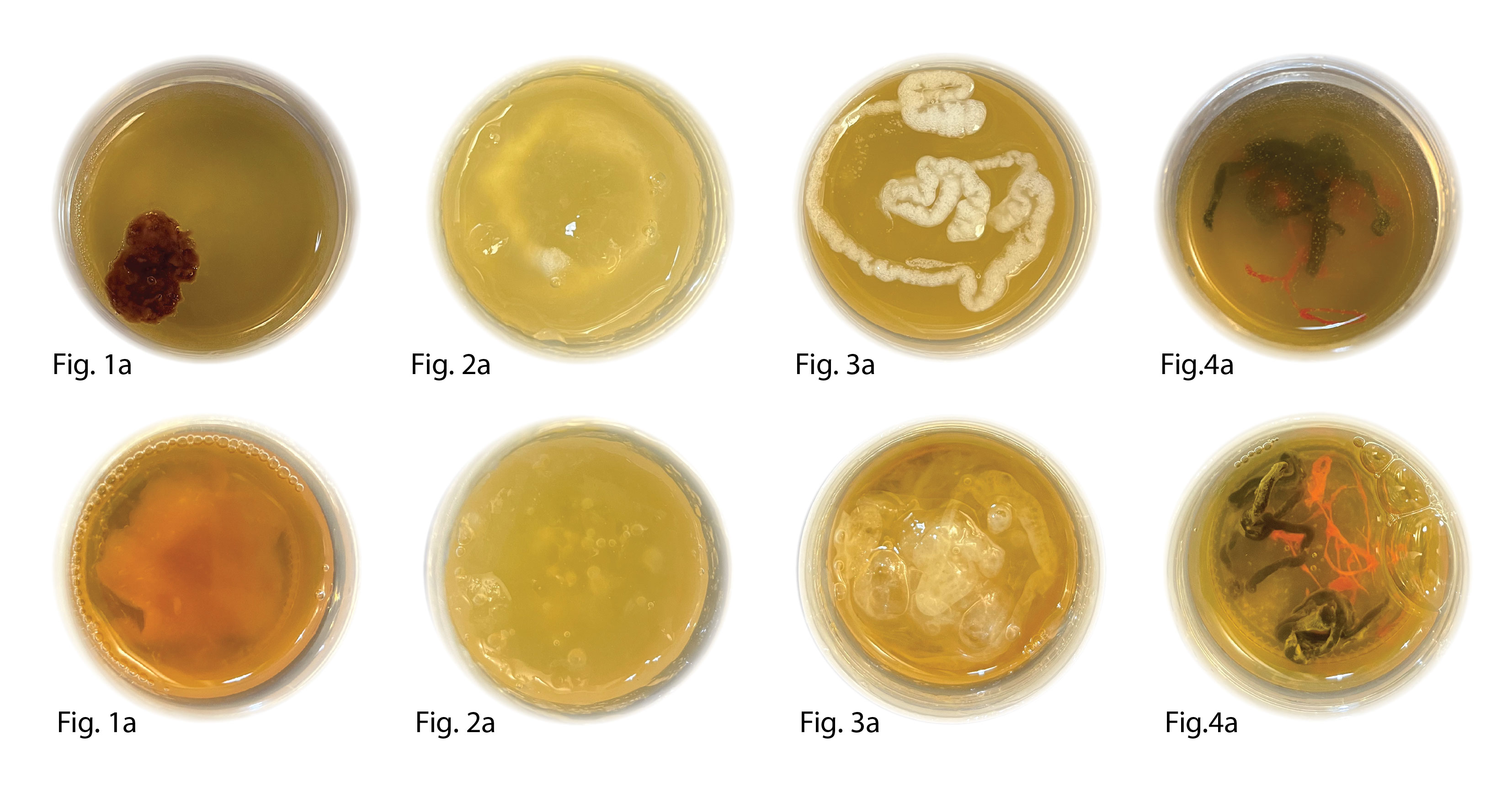
From left to righ: Fig.1a&b S-70 dried SCOBY+ Xantham, Fig.2 a&b S-67 foamed BC ink+ alginate support bath, Fig.3a&b S-66 foamed BC ink+ gelatin support bath, Fig.4a&b Alginate and Chitosan scaffold in cultural media
Conclusion: Samples was prepared according to Excel sheet and left in a sealed plastic container for 1 week at 30°C.
The container for Sample 66, 67, 70 had significantly expanded indicating CO2 production.
Sample S-70 and S-66 had clear visual sign of growth wile 67 seamed to have dissolved into the support bath leaving few airbubbles in the support-bath. The chitosan scaffold didn't show much growth.
The microalgae experiments was not possible to conduct due to slow growth of the algae however the foaming using an ice-cream stabilizer significantly increased the stability of the foam. It could hold its volume during the 2 weeks test period without significant loss. However the cell growth was limited.
 overview of the 2nd round of samples launched
overview of the 2nd round of samples launched
Experiment 3 BC samples:¶
Several variations of earlier samples were relaunched using different SCOBY concentrations and ratios of polymer. Every sample also used fresh culture media recipes using instant coffee or green tea as a nitrogen source and sucrose from the supermarket. In some cases, a yeast extract was used to jumpstart the fermentation process.
The recipes were printed
1) In the support bath either in 1.5 % Xanthan gum or 4% w/v gelatin.
2) In a Petri dish with a covered lid
All samples were incubated for at least 2 weeks at 30°C
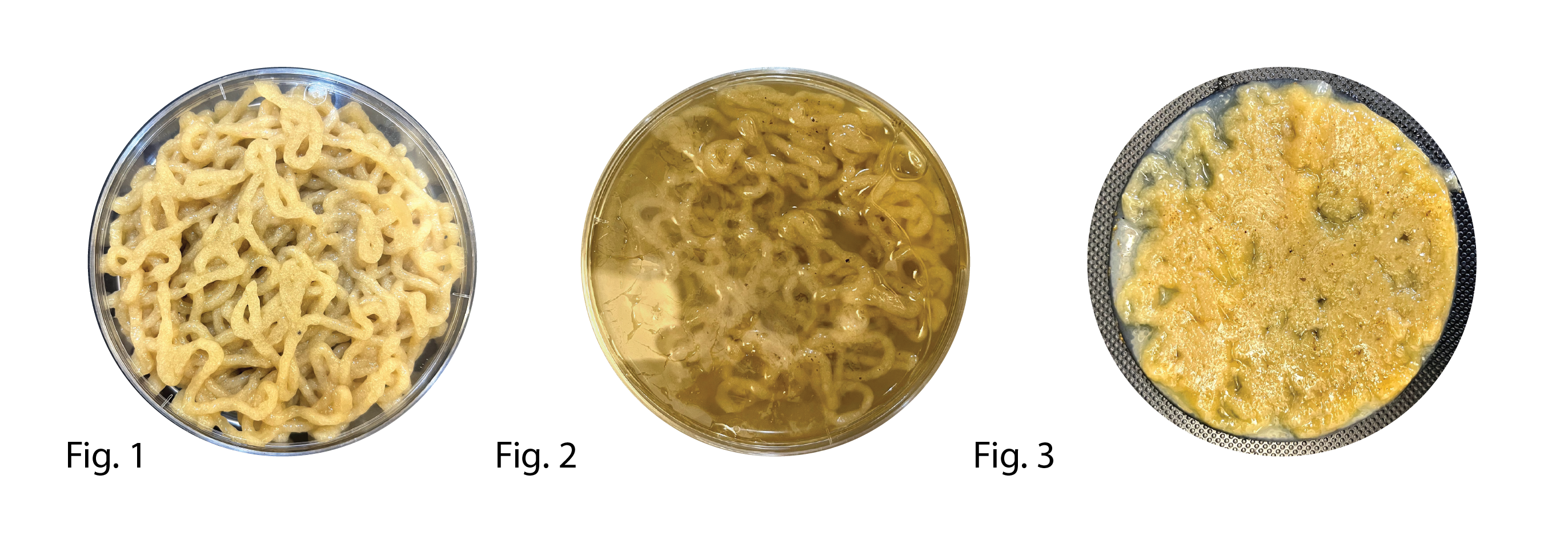 97 was printed using a hand syringe (fig.1) and after being printed covered with culture media (fig2.)
97 was printed using a hand syringe (fig.1) and after being printed covered with culture media (fig2.)
Fig 3. Most growth seemed to be rather around the print in the liquid culture media. The result was one of the strongest pellicles we managed to achieve.
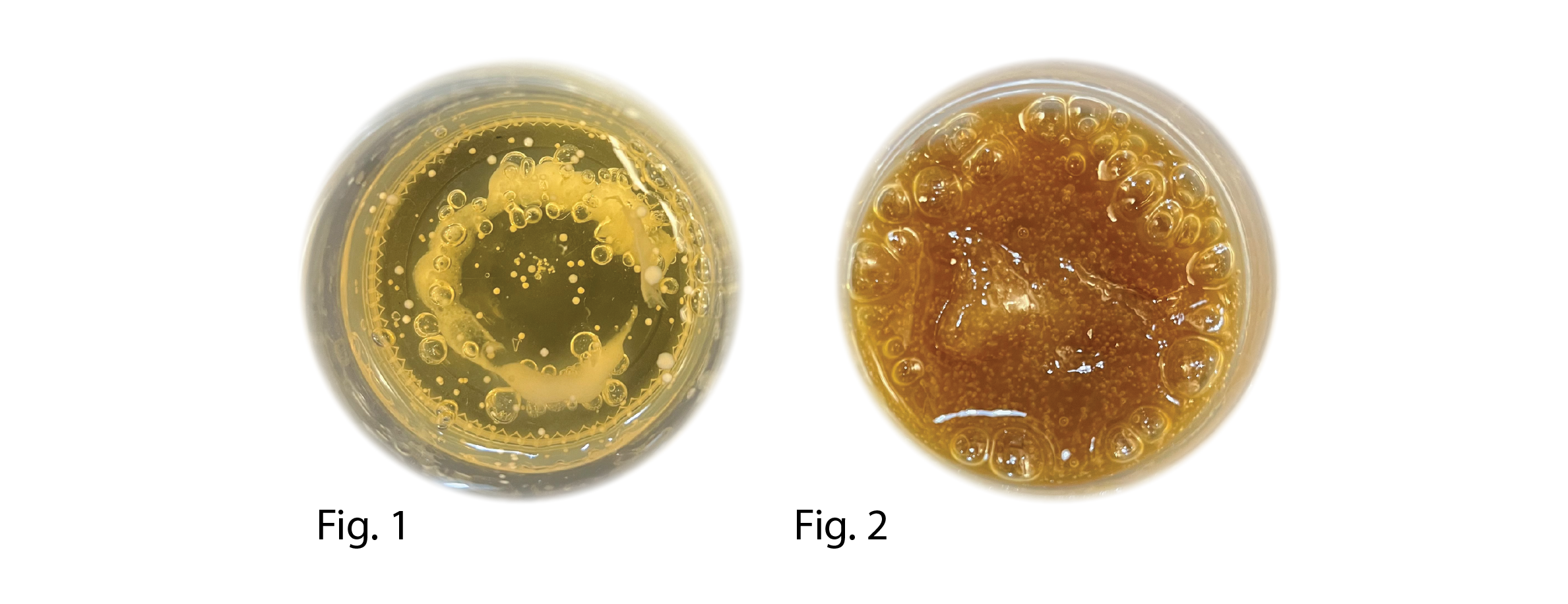 left S91 (printed in S.92) right S95
left S91 (printed in S.92) right S95
The jars swallowed up during the incubation period and clear signs of air bubbles indicated Co2 production, the print also seemed to gain in strength indicating cellulose production. However, the samples were too small to be able to analyze them further.
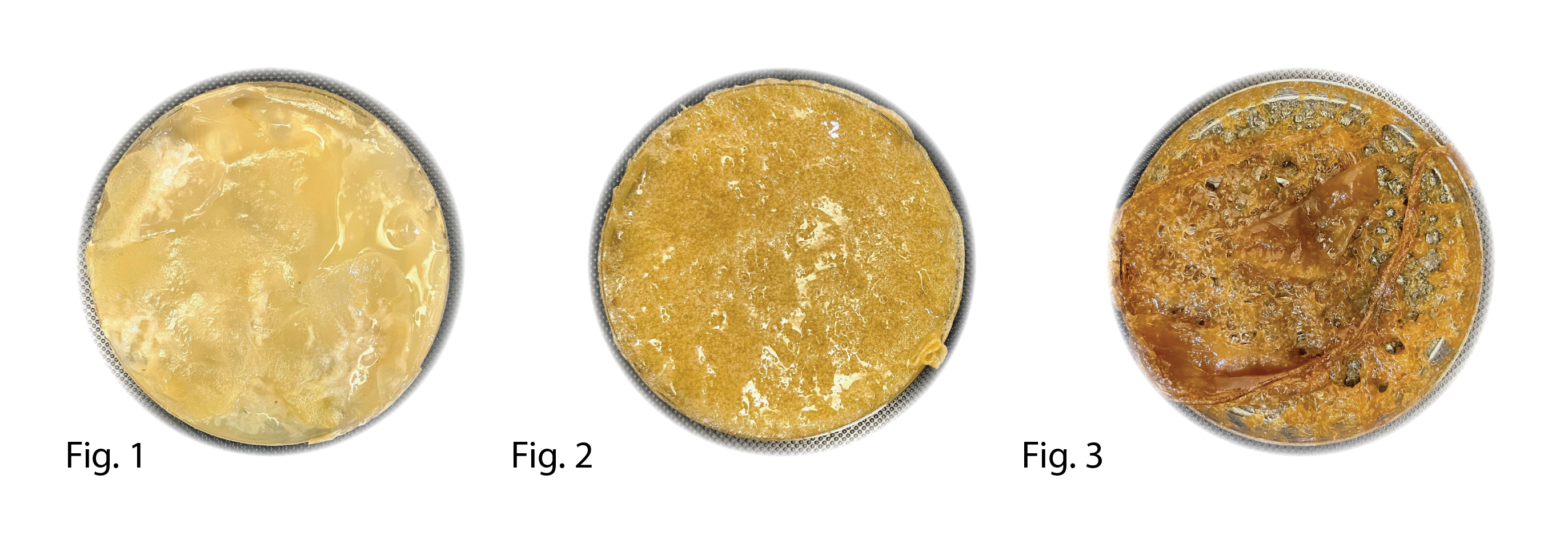 Fig1. 105b Fig2. 103c Fig3. 102 c
Fig1. 105b Fig2. 103c Fig3. 102 c
Many of the Petri dish samples didn't show any proof of growth and were falling apart when treated however samples were quite strong and showed some cellulose production.
 100a coffe/alginate scaffold Fig1 freshly printed fig2 after two weeks of incubation
100a coffe/alginate scaffold Fig1 freshly printed fig2 after two weeks of incubation
The scaffold experiments did not show any growth
 Co2 production in XG suspension bath
Co2 production in XG suspension bath
Reflection of the BC living-ink protocol:¶
Around 100 test was conducted trying to print bacterial cellulose harvested from a DIY kombucha fermentation process. We outlined some of the difficulties with oxygen supply for this process and suggested a few research studies tackling this problem. The experiments were conducted both as prints in suspension bath as well as in Petri dishes.
The reference research studies that were used as inspiration for this research was conducted with isolated BC strands and using expensive specialized culture medias. In this study we used simple DIY kombucha recipes that sometimes made the translation of the experiments difficult. We outlined a few different recipes using different culture media recipes, polymers and SCOBY concentrations. Because of the lack of some advanced lab equipment’s the results was often hard to analyze however some conclusions could be made.
The 3ed round of samples that contained a higher SCOBY concentration and lower polymer concentration seemed to have better growth. Also, the addition of fresh culture media indicated a longer growth time compared to earlier experiments. However, none of the samples showed a dense enough growth that they would be suitable to make garments out of. When purified after incubation time even the dense samples fell into pieces.
It is possible that further experimentation would give better results however it is I believe clear that this process shows a lot of problems and very few advantages compared to the purified roadmap. Most importantly hurdle is that the process is complex and requires a sterile lab environment that would make scaling the process for a fashion manufacturing process highly challenging.
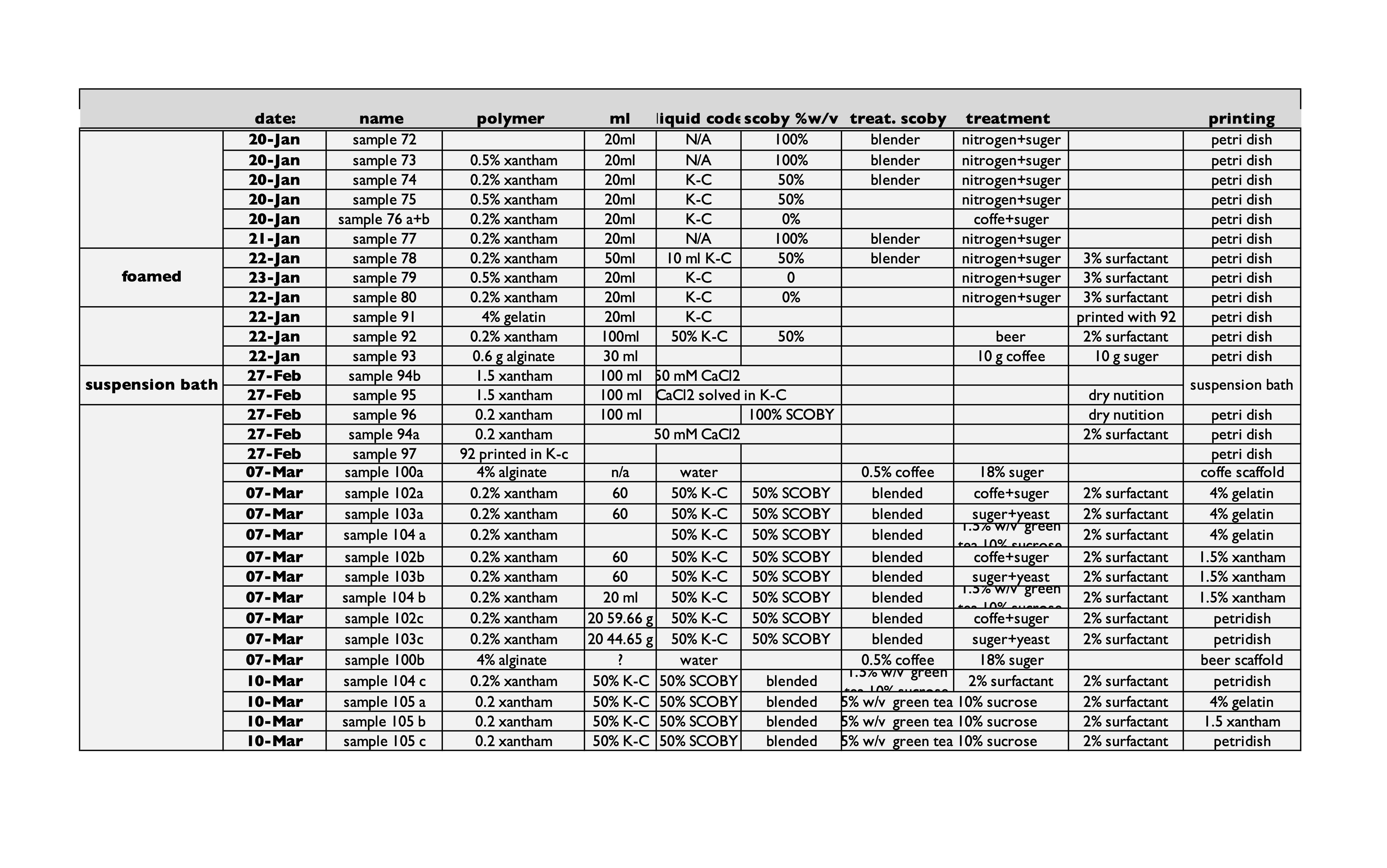 overview of the 3ed round of samples launched
overview of the 3ed round of samples launched