| III. - KERATIN |¶
KERATIN EXTRACTION METHODS¶
- METHODS
- 1. CHEMICAL
- 1.1. REDUCTION
- 1.1.1. thiol containing chemicals (_a._ L-Cysteine)
- 1.1.2. sulfitolysis (_a._ sodium sulphite, _b._ bisulfite, _c._ disulfite)
- 1.2. OXIDINATION
- 1.2.1. Paracetic acid
- 1.2.2. Performic acid
- 1.3. HYDROLYSIS
- 1.3.1. Sodium hydroxide
- 1.4. IONIC LIQUIDS
- 1.4.1. 1-Allyl-3-methylimidazolium chloride
- 1.4.2. 1-Butyl-3-methylimidazolium chloride
- 1.4.3. 1-Butyl-3-methylimidazolium tetrafluoroborate
- 1.4.4. 1-Butyl-3-methylimidazolium hexafluorophosphate
- 1.4.5. 1-Butyl-3-methylimidazolium bromide
- 2. MICROBIAL & ENZYMATIC
- 2.1. Gram negative bacteria (_a._ Stenorophmonas SP, _b._ Chryseobacterium SP, _c._ Vibrio SP)
- 2.2. Gram positive bacteria (_a._ Bacillus SP, _b._ Kocuria rosea)
- 2.3. Saprohytic and parasitic fungi
- 3. SUPERCRITICAL WATER AND STEAM EXPLOSION
- 4. MICROWAVE IRRADIATION
- SOURCE: Keratin: dissolution, extraction and biomedical application, Amin Shavandi, Tiago H. Silva, Adnan A. Bekhit and Alaa El-Din A. Bekhit, 2017, DOI: 10.1039/c7bm00411g, rsc.li/biomaterials-science
REDUCTION¶
- is using reducing agents as thiols
- has been the most reported technique to break the cystine disulfide bonds
- disadvantages of being expensive and can be toxic and harmful
- Sulfitolysis - sodium sulfide - 1. sulfitolysis step, 2. cysteine-sulfonate - both require the use of large amounts of urea as a protein denaturant, which can change the physicochemical properties of the final keratin.
OXIDATION¶
- with oxidizing materials such as formic or peracetic acids being the most frequently used acids to form a sulfonic acid
- a time-consuming process with more than 24 h
HYDROLYSIS¶
- Alkali extraction - requires amounts of alkaline chemicals for hydrolysis and acids for neutralization
- the primary chain of keratin is damaged
IONIC LIQUIDS¶
-
New green solvents - this process needs to be carried out under nitrogen, requires a precise temperature control, the raw material needs to be added in small portions to the hot liquid, and the obtained keratin is not water soluble.
-
SOURCE: Keratin-based Biomaterials and Bioproducts, _Narendra Reddy, 2017, ISBN: 978-1-91024-287-2, http://www.polymer-books.com
L-CYSTEINE¶
- L-Cysteine is an α-amino acid with the chemical formula HOOCCH(NH2)CH2SH. Due to the ability of thiols to undergo redox reactions, we used L-cysteine as a substitute for 2-mercaptoethanol which is a benchmark for extracting keratin because of good yield and undamaged keratin, but causes environmental harmfulness.
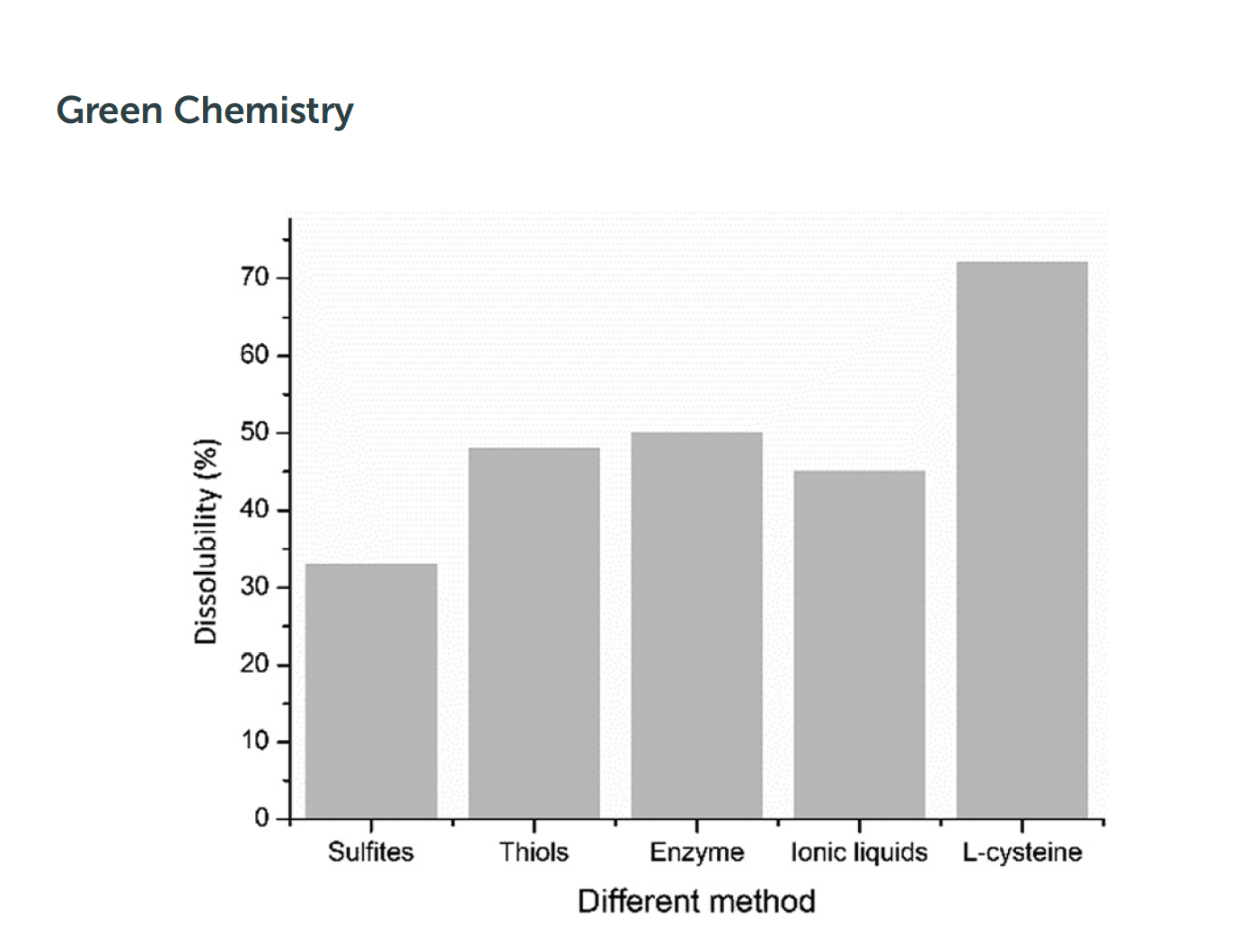
- SOURCE: Extracting keratin from wool by using L-cysteine, K. Wang,a R. Li, J. H. Ma, Y. K. Jiana and J. N. Cheb, 2015, DOI: 10.1039/c5gc01254f, www.rsc.org/greenchem
Last update: 2022-05-10