4. Biochromes¶
Here are some definitions, concepts and techniques we learnt during the lecture that I feel are going to be essential to this week:
Biochromes: Colors produced by organisms.
Ink: Thick liquid dense in colour. It can be soluble or dispersed.
Dyes bond to the material they get on. They are soluble in water and are often in the form of a liquid bath.
Pigment: powder insoluble in water.
The following chart represents the cycle of pigments and how they can be extracted from the natural source, then transformed into inks and dyes
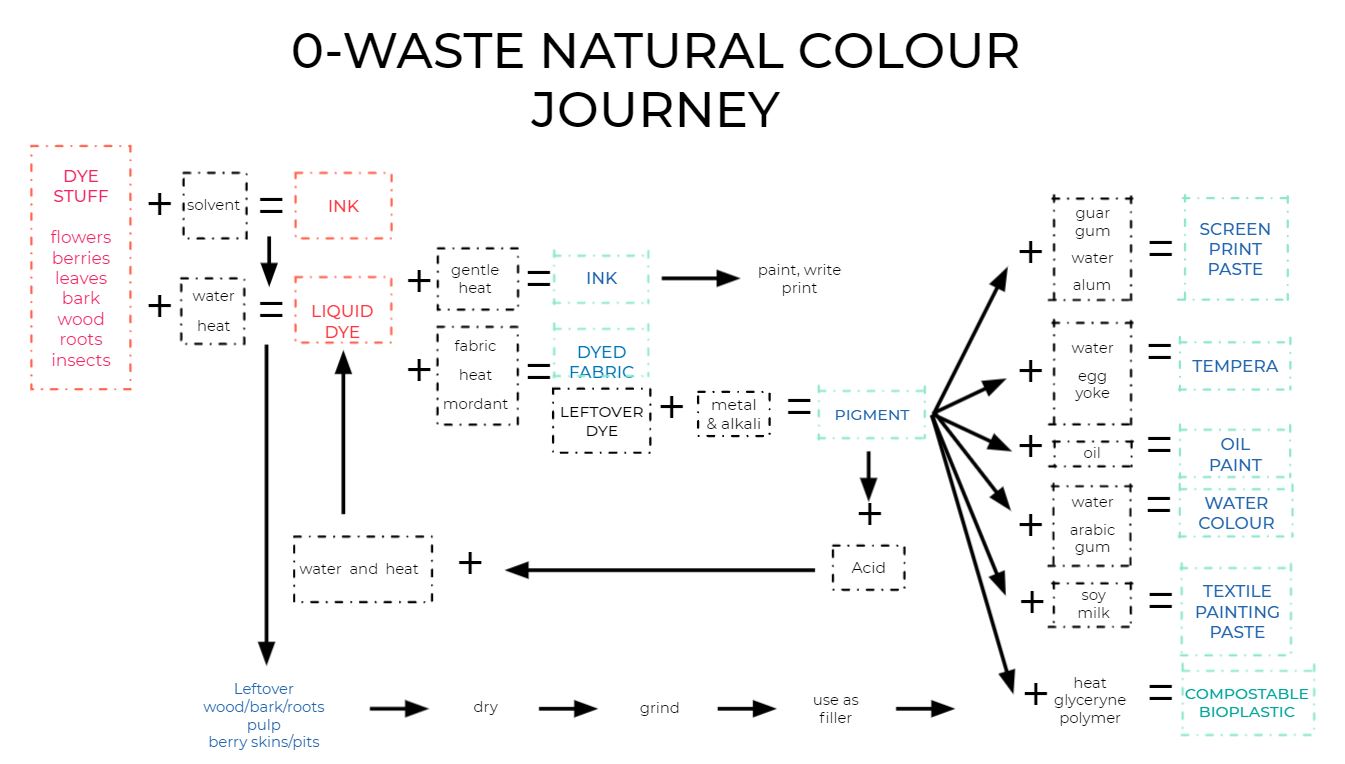
Assignment¶
-
Produce at least one natural dye or bacterial dye: Natural dye - modifying its colour and mordanting it in different ways to dye at least 2 different categories of fibers Bacterial dye - Explored dyeing with bacteria of different fibers and/or bacteria
-
Produce at least one botanical or bacterial ink: Botanical Ink - explore different materials, recipes and understand how to adjust them based on the ingredients Bacterial Ink - explore bacteria solvents and understand how to adjust or use the inks
I-Natural plant-based dyes.¶
Dyeing processes vary depending on the fabric to be dyed, the dye used and the final color intensity you want. For this assignement, each and everyone of us had to dye three types of fabric with a natural dye. The fabrics were cotton (plant fibers, composed of cells and cellulose), silk (animal fibers, composed of cells and proteins) and viscose rayon (reconstructed fibers made from cellulose extracted from plants). My assigned plant-based dye was onion peels, which can produce a range of fibers from yellow/orange to olive green. After being assigned a plant-base-dye, each one of us had to do some research about the dyeing process.

1-Scouring (necessary to all types of fibers) [1][2].¶
The scouring process allows to clean the fibers from dirt but also oils, waxes and other components covering the fabric when they are produced and might prevent the pigments from attaining the fibers.
-
Animal fibers For this step, add the fibers in a pot with 0,5-1% weight of fabric (WOF) natural detergent and 1-5% WOF soda ash. Fill the pot with water and stir well. Make sure to put enough water so that the fibers can float freely. Slowly bring the temperature to 80-85°C and hold for 30 min. Remove fibers and rinse. Let it soak in water and 5% WOF vinegar for 20 min and rinse again.
-
Plant and cellulose fibers In a pot, add the fibers and 2% WOF soda ash. Fill the pot with water and stir well. Make sure to put enough water so that the fibers can float freely. Slowly bring the temperature to 80-85°C and hold for 30 min. Remove fibers and rinse.
In Fabricademy Barcelona, we did the dyeing all together and on three different fabrics: cotton (plant), viscose (cellulose from plants) and silk (animal). As we had a lot of pieces of fabric to dye, we decided to apply the same scouring technique to all fabrics: we used 2% WOF soda ash and simmered the fabrics for at least 1 hour.

2-Mordanting (optional when using onion peels and other natural dyes rich in tannins, and useless on animal fibers) [1][3]¶
During this step, the fibers are going to be opened by a metal solution (Alum salt, Copper or Iron) which are going to allow the pigments to properly enter the fibers. When the natural dye is rich in tannins, the tannins achieve the same role as the metal solution so the mordanting step is unnecessary. Soy can also be used in this step but it is a binder, not a mordant.
- In a pot, add scoured fibers, 10-25% WOF mordant (make sure to dilute the mordant powder in water before) and fill with water. Stir well and heat at 70°C for 60 min. Rinse well. As we performed the mordanting steps all together, we decided to mordant half of each fabric with Alum salt and the other half with Iron sulfate. We used 12% WOF when using the Aluminum mordant and 1% WOF when using the iron mordant. Copper was not used as it presents more health hazards.

3-Dyeing [4] [5] [6] [7]¶
This step depends on the type of dye used. In the case of onion peels, one should simmer 25% WOF onion skins in a pot filled with water for 30 to 60 minutes. For a deeper color, it can be let to cool in the pot overnight. Then the onion peels should be strained and the dye bath is ready to be used. Simmering the fabric in the dye bath for 40 to 60 minutes will dye the fabric in a range of colors from yellow to orange. The fabric can be put to dry and then rinsed or rinsed right away, depending on the desired intensity of color.

For the onion skins dye, I made two bath: one for the material previously mordanted with iron and the other one with the material previously mordanted with alum. I put each material to simmer in the bath for 60, 50 and 40 minutes to see if I would get different intensities of color, but only got the same shades for each material. For the next time, I should try 20, 40 and 60 minutes and maybe I will get differences in the intensity. The pH of the onion dye was acidic, around 4. The samples are displayed in the following order : cotton - viscose - silk.


4-Modifying [1] [6]¶
The color of the dye can be modified by various molecules allowing a broader range of colors for each dye. The modifier can be added in the dye bath during the dyeing step, or on the fabric after it has been dyed. In some cases, the modifier is also a mordant so using it during the mordanting step will directly lead to a modified color on the fabric. There are different types of modifyers: * Iron: iron, when added to the dye bath, lead to a darker color. With onion peels, the color switches from orange to dark brown. * When the pH of the onion peels dye bath is too alkaline, the color changes from orange to yellow. For a deeper orange, add an acid (vinegar of lemon juice).
For my fabric, I had already tried the modifyer with the Iron sulfate during the mordanting step. I then decided to try acid and alkaline solutions on the dyed fabrics. With a brush, I painted an alkaline solution of bakind soda on the viscose fabric and obtained a darker shade of orange (which is quite surprising because I expected the color to be more yellow). Adding vinegar (acid) did not change the color. These results are quite dissapointing but I blame it on the fact that I put the modifyer on a dyed and dry fabric and suppose it would have been different if I added the modifyers in the dye bath directly.

5-Recycling of the dye: precipitation of the pigments¶
As presented in the first image of this page, pigments and dyes follow a cycle and once we are done dyeing the fabrics, the remaining pigments can be precipitated and transformed in paints or inks. To precipitate and then separate the pigments from the other components of the dye bath, one needs to mix 1 portion of dye bath with two portions of acid (alum solution). Then, the addition of one portion of base (baking soda solution) will make the pigments precipitate. This reaction generates foam and is exothermic so be careful!


6- Other dyes¶
As I did the onion peels dye, the other members of Fabricademy Barcelona tried other natural components. They are listed in the scheme below, alongside with their dyeing characteristics.
| POMEGRANATE | TURMERIC | BLACK BEANS | RED CABBAGE | EUCALYPTUS | TARA PODS | AVOCADO PITS |
|---|---|---|---|---|---|---|
| Lightfast | Lightfast | Lightfast | Fugitive | Fugitive | Lightfast | Medium Lightfast |
| Yellow fawn | Yellow | Purple | Purple | Yellow/Green | Intense orange | Peach |
| Iron mordant: golden yellow | Iron mordant: brown | Iron mordant: darker purple | Iron mordant: darker purples | Iron mordant: orange/red, green/brown, black/grey | Iron mordant: black/purple/grey | Iron mordant: brown |
| High in tannins | High in tannins | - | pH sensitive | - | - | pH sensitive |
| No recommendation | 1/8 WOF | No recommendation | No recommendation | 1/6 WOF | 1/2 WOF | No recommendation |
Some colors are called fugitive colors because they fade away when exposed to the light, like cabage. The resistance of the color can be measured on a scale of lightfastness: the more lightfast the color, the longer the pigments stay.
Here is a picture of all the color shades we were able to obtain and I think it is amazing! I really love the dark brown I obtained with the onion skins and iron on the viscose fabrics and the light pastel pinks obtained with the avocado pits, but my personal favorite is the super bright yellow obtained from the tumeric.


II-Bacterial dyes¶
Some bacteria have developped the ability to produce pigments as defense mechanisms against other microorganisms as the pigments sometimes have antimicrobial proprieties. It is the case of Janthinobacterium lividium producing violacein, a purple pigment, or Serratia marcensces producing prodigiosin, an orange/red pigment. Growing these bacteria on fabric allow dyeing the material with different patterns depending on where the bacteria are initially placed and how they grow.
1-Growth medium¶
These two bacteria grow on LB medium (lysogeny broth), which is a medium providing peptides, vitamins, oligo elements and minerals necessary to most bacteria to grow. This medium can be prepared liquid or solid, depending on the addition of agar to gelify it (sometimes the medium needs to be solid because some bacteria need a support to grow and can not grow dispersed in a liquid).
-
J. lividium. At Fabricademy Barcelona, we only had J. lividium and this bacterium produces violacein optimally at 25°C, pH 7 and with 1% v/v glycerol [8]. When preparing liquid LB to grow it in Fabricademy, we used ready made LB powder and used the proportions of 0,025 w/v (weight by volume, in g/mL, that is to say 25 grams of in one liter of deionized water) and 4% v/v glycerol. Since I read J. lividium produces more violacein when it has glycerol, I also prepared a LB medium with no glycerol to make different intensities of purple once the bacteria grow on it. For the agar LB, we used the proportions of 0,025 w/v LB powder and 0,015 w/v agar powder. The LB agar was used to cast petri dishes and spread the bacteria again to keep it alive. The liquid LB was used to bring nutrients to the bacteria when we made it grow on our material.
-
S. marcensces. Just as J. lividium, this bacterium can also grow and produce prodigiosin on LB medium (agar or liquid). It grows at temperatures up to 32°C, in a large range of pH and is resistant to ampicilin [9]. We did not have this bacterium at Fabricademy barcelona but according to this article, the prodigiosin production is inhibited by gluconate and inhibited by glucose. An interesting experiment could be to make this bacterium grow in liquid LB, and give it a fabric as a support (the support would be immersed in the petri dish) and then, in some spot, put droplets of glucose and in others, put droplets of gluconate. That way, in some spots, the bacteria would produce more prodigiosin and in others, they would not, giving different intensities of dye.

When doing some scientific research on these bacteria and the pigment production, I found this iteresting article from Scloss, 2010 who explains the discovery of a J. lividium strain capable of producing prodigiosin at low temperatures (around 6°C). This strain does not produce violacein but it could be interesting to grow that strain at the same time as the one producing violacein and make different patterns and colors depending on the temperature we expose it to. Of course, the production of both colors can also be achieved by making a co-culture of J. lividium and S. marcensces but these two organisms are from different species so it would be more difficult than having only one, and the red color production would not be related to the temperature.
2-Sterile environment¶
LB medium is a medium allowing the growth of a large range of bacteria. Thus, if we do not work in a sterile environment, a lot of contaminant bacteria and fungus we are not interested in might grow on the plates and contaminate our J. lividium culture. This is why we have to work in a sterile environment. In a lab, to make glassware sterile, we use an autoclave which kills most of the microorganisms and spores by elevating the temperature to 134°C for 18 min. The exact same mechanism can be achieved in a pressure cooker. At fabricademy Barcelona, we used it to sterilize our petri dishes in which we had put some silk folded in the shape we desired and the LB mediums we prepared. In order to maintain a sterile environment, we cleaned the workspace with ethanol and we used a camping gas to create a sterile environment in the air (the fire burns and kills the bacteria, and the hot air is lighter than the cold air so it goes up, creating a sterile zone). That way, we were able to inoculate the petri dishes with the bacteria.

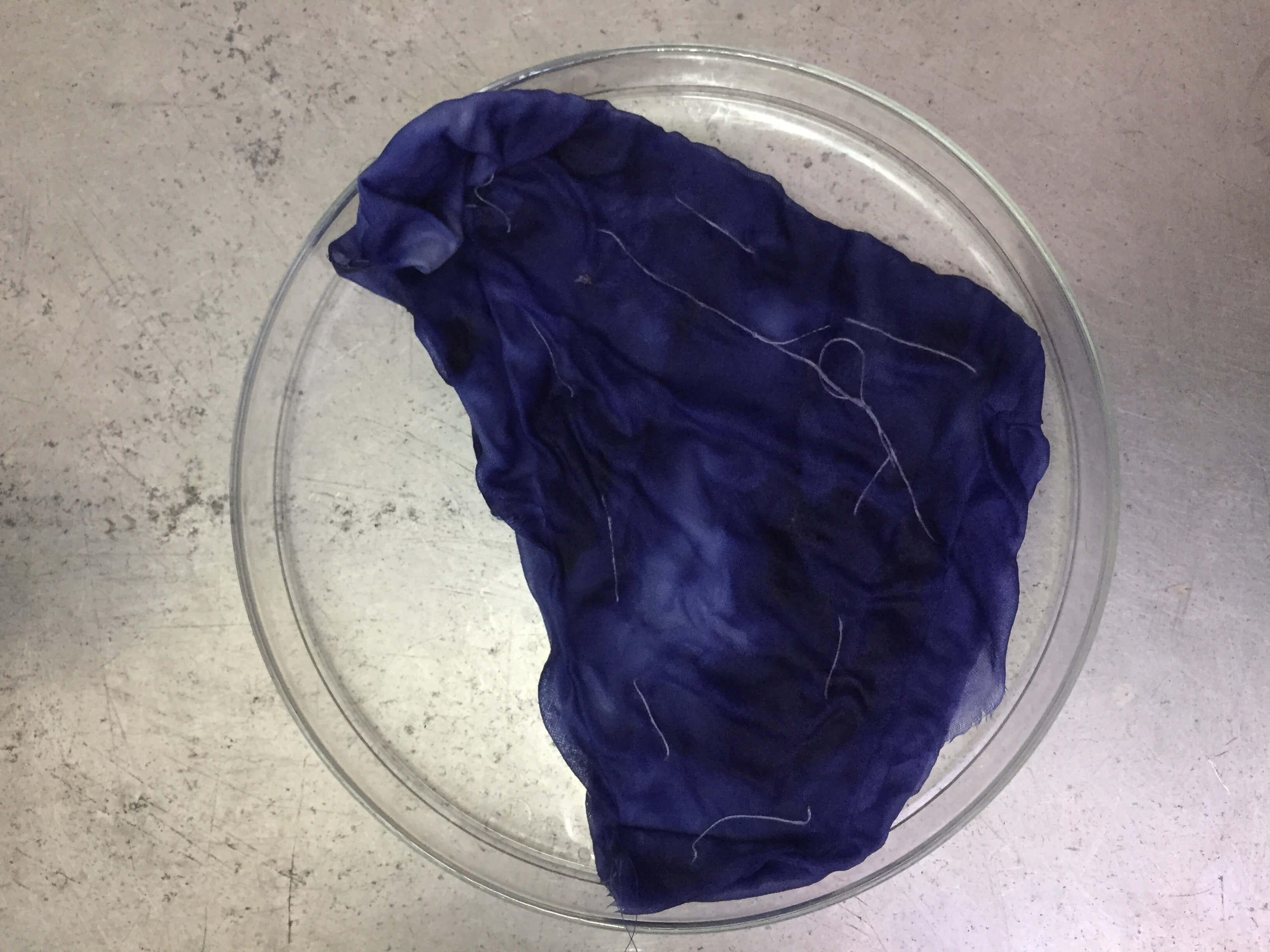
3-Manipulating the bacteria¶
What I did was, in the sterile envrionment, take an empty petri dish, fill it with the LB medium without glucose and with an anse that I sterilized in the flames of the camping gas, I diluted some bacteria in it. I poured the mix of LB and J. lividium in the petri dish containing my folded silk fabric. Then, in some spots, I poured some LB+glycerol on some spots of the petri dish. Then with the sterile anse, I spread some bacteria directly in some spots of the fabcric, creating another pattern (in case the one with glycerol does not function).
